Abstract
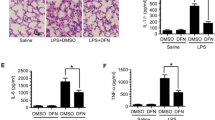
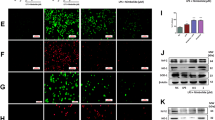
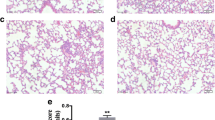
Endotoxemia induced by lipopolysaccharide (LPS) is an extremely severe syndrome identified by global activation of inflammatory responses. Neutrophil extracellular traps (NETs) play an important role in the development of endotoxemia. Histone hypercitrullination catalyzed by peptidylarginine deiminases (PADs) is a key step of NET formation. We have previously demonstrated that simultaneous inhibition of PAD2 and PAD4 with pan-PAD inhibitors can decrease NETosis and improve survival in a mouse model of LPS-induced endotoxic shock. However, the effects of PAD2 specific inhibition during NETosis and endotoxic shock are poorly understood. Therefore, in the present study, we aimed to investigate the effect of the specific PAD2 or PAD4 inhibitor on LPS-induced endotoxic shock in mice. We found that PAD2 inhibition but not PAD4 inhibition improves survival. Also, the levels of proinflammatory cytokines and NETosis were significantly reduced by PAD2 inhibitor. To our knowledge, this study demonstrates for the first time that PAD2 inhibition can reduce NETosis, decrease inflammatory cytokine production, and protect against endotoxin-induced lethality. Our findings provided a novel therapeutic strategy for the treatment of endotoxic shock.





Similar content being viewed by others
References
Kulp, A., and M.J. Kuehn. 2010. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annual Review of Microbiology 64: 163–184. https://doi.org/10.1146/annurev.micro.091208.073413.
Opal, S.M. 2010. Endotoxins and other sepsis triggers. Contributions to Nephrology 167: 14–24. https://doi.org/10.1159/000315915.
Ramnath, R.D., S.W. Ng, A. Guglielmotti, and M. Bhatia. 2008. Role of MCP-1 in endotoxemia and sepsis. International Immunopharmacology 8 (6): 810–818. https://doi.org/10.1016/j.intimp.2008.01.033.
Grigoleit, J.S., J.R. Oberbeck, P. Lichte, P. Kobbe, O.T. Wolf, T. Montag, A. del Rey, E.R. Gizewski, H. Engler, and M. Schedlowski. 2010. Lipopolysaccharide-induced experimental immune activation does not impair memory functions in humans. Neurobiology of Learning and Memory 94 (4): 561–567. https://doi.org/10.1016/j.nlm.2010.09.011.
Hirz, T., and C. Dumontet. 2016. Neutrophil isolation and analysis to determine their role in lymphoma cell sensitivity to therapeutic agents. Journal of Visualized Experiments 109: e53846. https://doi.org/10.3791/53846.
Yipp, B.G., and P. Kubes. 2013. NETosis: How vital is it? Blood 122 (16): 2784–2794. https://doi.org/10.1182/blood-2013-04-457671.
Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D.S. Weiss, Y. Weinrauch, and A. Zychlinsky. 2004. Neutrophil extracellular traps kill bacteria. Science 303 (5663): 1532–1535. https://doi.org/10.1126/science.1092385.
Camicia, G., R. Pozner, and G. de Larranaga. 2014. Neutrophil extracellular traps in sepsis. Shock 42 (4): 286–294. https://doi.org/10.1097/shk.0000000000000221.
Wong, S.L., M. Demers, K. Martinod, M. Gallant, Y. Wang, A.B. Goldfine, C.R. Kahn, and D.D. Wagner. 2015. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nature Medicine 21 (7): 815–819. https://doi.org/10.1038/nm.3887.
Li, R.H.L., G. Ng, and F. Tablin. 2017. Lipopolysaccharide-induced neutrophil extracellular trap formation in canine neutrophils is dependent on histone H3 citrullination by peptidylarginine deiminase. Veterinary Immunology and Immunopathology 193-194: 29–37. https://doi.org/10.1016/j.vetimm.2017.10.002.
Wang, S., and Y. Wang. 2013. Peptidylarginine deiminases in citrullination, gene regulation, health and pathogenesis. Biochimica et Biophysica Acta 1829 (10): 1126–1135. https://doi.org/10.1016/j.bbagrm.2013.07.003.
Wang, Y., M. Li, S. Stadler, S. Correll, P. Li, D. Wang, R. Hayama, L. Leonelli, H. Han, S.A. Grigoryev, C.D. Allis, and S.A. Coonrod. 2009. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. The Journal of Cell Biology 184 (2): 205–213. https://doi.org/10.1083/jcb.200806072.
Zhang, X., M. Bolt, M.J. Guertin, W. Chen, S. Zhang, B.D. Cherrington, D.J. Slade, C.J. Dreyton, V. Subramanian, K.L. Bicker, P.R. Thompson, M.A. Mancini, J.T. Lis, and S.A. Coonrod. 2012. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor alpha target gene activation. Proceedings of the National Academy of Sciences of the United States of America 109 (33): 13331–13336. https://doi.org/10.1073/pnas.1203280109.
Liang, Y., B. Pan, H.B. Alam, Q. Deng, Y. Wang, E. Chen, B. Liu, Y. Tian, A.M. Williams, X. Duan, Y. Wang, J. Zhang, and Y. Li. 2018. Inhibition of peptidylarginine deiminase alleviates LPS-induced pulmonary dysfunction and improves survival in a mouse model of lethal endotoxemia. European Journal of Pharmacology 833: 432–440. https://doi.org/10.1016/j.ejphar.2018.07.005.
Martinod, K., T.A. Fuchs, N.L. Zitomersky, S.L. Wong, M. Demers, M. Gallant, Y. Wang, and D.D. Wagner. 2015. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 125 (12): 1948–1956. https://doi.org/10.1182/blood-2014-07-587709.
Muth, A., V. Subramanian, E. Beaumont, M. Nagar, P. Kerry, P. McEwan, H. Srinath, K. Clancy, S. Parelkar, and P.R. Thompson. 2017. Development of a selective inhibitor of protein arginine deiminase 2. Journal of Medicinal Chemistry 60 (7): 3198–3211. https://doi.org/10.1021/acs.jmedchem.7b00274.
Park, S.Y., S. Shrestha, Y.J. Youn, J.K. Kim, S.Y. Kim, H.J. Kim, S.H. Park, W.G. Ahn, S. Kim, M.G. Lee, K.S. Jung, Y.B. Park, E.K. Mo, Y. Ko, S.Y. Lee, Y. Koh, M.J. Park, D.K. Song, and C.W. Hong. 2017. Autophagy primes neutrophils for neutrophil extracellular trap formation during sepsis. American Journal of Respiratory and Critical Care Medicine 196 (5): 577–589. https://doi.org/10.1164/rccm.201603-0596OC.
Deng, Q., T. Zhao, B. Pan, I.S. Dennahy, X. Duan, A.M. Williams, B. Liu, N. Lin, U.F. Bhatti, E. Chen, H.B. Alam, and Y. Li. 2018. Protective effect of tubastatin A in CLP-induced lethal sepsis. Inflammation 41 (6): 2101–2109. https://doi.org/10.1007/s10753-018-0853-0.
Gotts, J.E., and M.A. Matthay. 2016. Sepsis: Pathophysiology and clinical management. Bmj 353: i1585. https://doi.org/10.1136/bmj.i1585.
Pan, B., H.B. Alam, W. Chong, J. Mobley, B. Liu, Q. Deng, Y. Liang, Y. Wang, E. Chen, T. Wang, M. Tewari, and Y. Li. 2017. CitH3: A reliable blood biomarker for diagnosis and treatment of endotoxic shock. Scientific Reports 7 (1): 8972. https://doi.org/10.1038/s41598-017-09337-4.
Gloude, N.J., P. Khandelwal, N. Luebbering, D.T. Lounder, S. Jodele, M.N. Alder, A. Lane, A. Wilkey, K.E. Lake, B. Litts, and S.M. Davies. 2017. Circulating dsDNA, endothelial injury, and complement activation in thrombotic microangiopathy and GVHD. Blood 130 (10): 1259–1266. https://doi.org/10.1182/blood-2017-05-782870.
van den Boogaard, M., B.P. Ramakers, N. van Alfen, S.P. van der Werf, W.F. Fick, C.W. Hoedemaekers, M.M. Verbeek, L. Schoonhoven, J.G. van der Hoeven, and P. Pickkers. 2010. Endotoxemia-induced inflammation and the effect on the human brain. Critical Care 14 (3): R81. https://doi.org/10.1186/cc9001.
Okeke, E.B., Z. Mou, N. Onyilagha, P. Jia, A.S. Gounni, and J.E. Uzonna. 2017. Deficiency of phosphatidylinositol 3-kinase delta signaling leads to diminished numbers of regulatory T cells and increased neutrophil activity resulting in mortality due to endotoxic shock. Journal of Immunology 199 (3): 1086–1095. https://doi.org/10.4049/jimmunol.1600954.
Stiel, L., F. Meziani, and J. Helms. 2018. Neutrophil activation during septic shock. Shock 49 (4): 371–384. https://doi.org/10.1097/SHK.0000000000000980.
Delabranche, X., L. Stiel, F. Severac, A.C. Galoisy, L. Mauvieux, F. Zobairi, T. Lavigne, F. Toti, E. Anglès-Cano, F. Meziani, and J. Boisramé-Helms. 2017. Evidence of netosis in septic shock-induced disseminated intravascular coagulation. Shock 47 (3): 313–317. https://doi.org/10.1097/SHK.0000000000000719.
Czaikoski, P.G., J.M. Mota, D.C. Nascimento, F. Sonego, F.V. Castanheira, P.H. Melo, G.T. Scortegagna, et al. 2016. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One 11 (2): e0148142. https://doi.org/10.1371/journal.pone.0148142.
Pieterse, E., N. Rother, C. Yanginlar, L.B. Hilbrands, and J. van der Vlag. 2016. Neutrophils discriminate between lipopolysaccharides of different bacterial sources and selectively release neutrophil extracellular traps. Frontiers in Immunology 7: 484. https://doi.org/10.3389/fimmu.2016.00484.
Gupta, S., and M.J. Kaplan. 2016. The role of neutrophils and NETosis in autoimmune and renal diseases. Nature Reviews. Nephrology 12 (7): 402–413. https://doi.org/10.1038/nrneph.2016.71.
Porto, B.N., and R.T. Stein. 2016. Neutrophil extracellular traps in pulmonary diseases: Too much of a good thing? Frontiers in Immunology 7: 311. https://doi.org/10.3389/fimmu.2016.00311.
McDonald, B., R.P. Davis, S.J. Kim, M. Tse, C.T. Esmon, E. Kolaczkowska, and C.N. Jenne. 2017. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129 (10): 1357–1367. https://doi.org/10.1182/blood-2016-09-741298.
Shen, X.F., K. Cao, J.P. Jiang, W.X. Guan, and J.F. Du. 2017. Neutrophil dysregulation during sepsis: An overview and update. Journal of Cellular and Molecular Medicine 21 (9): 1687–1697. https://doi.org/10.1111/jcmm.13112.
Kaufman, T., D. Magosevich, M.C. Moreno, M.A. Guzman, L.P. D'Atri, A. Carestia, M.E. Fandino, C. Fondevila, and M. Schattner. 2017. Nucleosomes and neutrophil extracellular traps in septic and burn patients. Clinical Immunology 183: 254–262. https://doi.org/10.1016/j.clim.2017.08.014.
Sakurai, K., T. Miyashita, M. Okazaki, T. Yamaguchi, Y. Ohbatake, S. Nakanuma, K. Okamoto, et al. 2017. Role for neutrophil extracellular traps (NETs) and platelet aggregation in early sepsis-induced hepatic dysfunction. In Vivo 31 (6): 1051–1058. https://doi.org/10.21873/invivo.11169.
Jimenez-Alcazar, M., C. Rangaswamy, R. Panda, J. Bitterling, Y.J. Simsek, A.T. Long, R. Bilyy, et al. 2017. Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 358 (6367): 1202–1206. https://doi.org/10.1126/science.aam8897.
Biron, B.M., C.S. Chung, X.M. O'Brien, Y. Chen, J.S. Reichner, and A. Ayala. 2017. Cl-amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. Journal of Innate Immunity 9 (1): 22–32. https://doi.org/10.1159/000448808.
Leshner, M., S. Wang, C. Lewis, H. Zheng, X.A. Chen, L. Santy, and Y. Wang. 2012. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Frontiers in Immunology 3: 307. https://doi.org/10.3389/fimmu.2012.00307.
Kolaczkowska, E., C.N. Jenne, B.G. Surewaard, A. Thanabalasuriar, W.Y. Lee, M.J. Sanz, K. Mowen, G. Opdenakker, and P. Kubes. 2015. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nature Communications 6: 6673. https://doi.org/10.1038/ncomms7673.
Li, P., M. Li, M.R. Lindberg, M.J. Kennett, N. Xiong, and Y. Wang. 2010. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. The Journal of Experimental Medicine 207 (9): 1853–1862. https://doi.org/10.1084/jem.20100239.
Rohrbach, A.S., D.J. Slade, P.R. Thompson, and K.A. Mowen. 2012. Activation of PAD4 in NET formation. Frontiers in Immunology 3: 360. https://doi.org/10.3389/fimmu.2012.00360.
Asaga, H., K. Nakashima, T. Senshu, A. Ishigami, and M. Yamada. 2001. Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils. Journal of Leukocyte Biology 70 (1): 46–51.
Takahara, H., M. Tsuchida, M. Kusubata, K. Akutsu, S. Tagami, and K. Sugawara. 1989. Peptidylarginine deiminase of the mouse. Distribution, properties, and immunocytochemical localization. The Journal of Biological Chemistry 264 (22): 13361–13368.
Horibata, S., K.E. Rogers, D. Sadegh, L.J. Anguish, J.L. McElwee, P. Shah, P.R. Thompson, and S.A. Coonrod. 2017. Role of peptidylarginine deiminase 2 (PAD2) in mammary carcinoma cell migration. BMC Cancer 17 (1): 378. https://doi.org/10.1186/s12885-017-3354-x.
Knight, J.S., V. Subramanian, A.A. O'Dell, S. Yalavarthi, W. Zhao, C.K. Smith, J.B. Hodgin, P.R. Thompson, and M.J. Kaplan. 2015. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Annals of the Rheumatic Diseases 74 (12): 2199–2206. https://doi.org/10.1136/annrheumdis-2014-205365.
Muller, S., and M. Radic. 2015. Citrullinated autoantigens: From diagnostic markers to pathogenetic mechanisms. Clinical Reviews in Allergy and Immunology 49 (2): 232–239. https://doi.org/10.1007/s12016-014-8459-2.
Mishra, N., L. Schwerdtner, K. Sams, S. Mondal, F. Ahmad, R.E. Schmidt, S.A. Coonrod, P.R. Thompson, M.M. Lerch, and L. Bossaller. 2019. Cutting edge: Protein arginine deiminase 2 and 4 regulate NLRP3 inflammasome-dependent IL-1beta maturation and ASC speck formation in macrophages. Journal of Immunology 203 (4): 795–800. https://doi.org/10.4049/jimmunol.1800720.
Ott, L.W., K.A. Resing, A.W. Sizemore, J.W. Heyen, R.R. Cocklin, N.M. Pedrick, H.C. Woods, J.Y. Chen, M.G. Goebl, F.A. Witzmann, and M.A. Harrington. 2007. Tumor necrosis factor-alpha- and interleukin-1-induced cellular responses: Coupling proteomic and genomic information. Journal of Proteome Research 6 (6): 2176–2185. https://doi.org/10.1021/pr060665l.
Funding
This work was funded by grants from Kickstart N022142 to Dr. Yongqing Li, UMHS-PUHSC Joint Institute U050150 and the National Institutes of Health Grant 5 R01 GM084127 to Dr. Hasan B. Alam and by the National Institutes of Health Grant R35 GM118112 to Paul R. Thompson.
Author information
Authors and Affiliations
Contributions
YL and HA designed the study. QD and BP carried out the experiments. YL and ZW wrote the manuscript, and YL and HA made a critical revision. QD, BP, YT, and UFB reviewed manuscript, and XD, BL, YT, and ZW provided experimental support. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The protocol for the animal experiments was approved by the University of Michigan Institutional Animal Care and Use Committee (PRO00008861). All experiments complied with animal welfare and research regulations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, Z., Deng, Q., Pan, B. et al. Inhibition of PAD2 Improves Survival in a Mouse Model of Lethal LPS-Induced Endotoxic Shock. Inflammation 43, 1436–1445 (2020). https://doi.org/10.1007/s10753-020-01221-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01221-0