Abstract
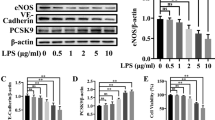
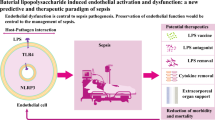
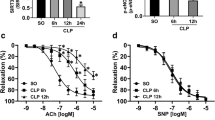
Endothelial dysfunction is responsible for multiple organ failure and the high mortality rate of sepsis. Nucleotide-binding domain-like receptor protein 3 (NLRP3) inflammasome plays an essential role in the progression of sepsis. However, the role of NLRP3 inflammasome in the endothelial dysfunction of sepsis has not been fully elucidated. In this study, septic mice were induced by cecal ligation and puncture (CLP) operation, and human umbilical vein endothelial cells (HUVECs) were treated with lipopolysaccharide (LPS). The 24-h survival rate after CLP was observed. Vasodilation function of the aorta was detected by vascular reactivity experiments. Expression of p-eNOS, eNOS, TLR4, MYD88, p-p65, p65, p-ikbα, ikbα, iNOS, NLRP3, and IL-1β in the aorta and HUVECs were determined by Western blot. Our results suggest that the p-eNOS expression was downregulated, the endothelium-dependent relaxation function was impaired, and TLR4, MYD88, p-p65, p-ikbα, iNOS, NLRP3, and IL-1β expression increased after CLP. The onset of death was 12 h after CLP, and the mortality rate was nearly 50% at 24 h after operation. The decline of p-eNOS, endothelium-dependent vasodilation function, and survival rate significantly improved with NLRP3-specific inhibitor MCC950 intervention or NLRP3 knockout in CLP mice. The decrease of p-eNOS in HUVECs induced by LPS was alleviated when pretreated with MCC950 or interleukin-1 receptor antagonist (IL-1Ra). In summary, our results indicate that activation of the NLRP3 inflammasome contributes to the development of endothelial dysfunction of early sepsis in mice, suggesting its potential role as a therapeutic target for the treatment of sepsis.






Similar content being viewed by others
References
Levy, B., S. Collin, N. Sennoun, N. Ducrocq, A. Kimmoun, P. Asfar, P. Perez, and F. Meziani. 2010. Vascular hyporesponsiveness to vasopressors in septic shock: from bench to bedside. Intensive Care Medicine 36: 2019–2029.
Angus, D.C., and T. van der Poll. 2013. Severe sepsis and septic shock. The New England Journal of Medicine 369: 840–851.
Rhodes, A., L.E. Evans, W. Alhazzani, M.M. Levy, M. Antonelli, R. Ferrer, A. Kumar, J.E. Sevransky, C.L. Sprung, M.E. Nunnally, B. Rochwerg, G.D. Rubenfeld, D.C. Angus, D. Annane, R.J. Beale, G.J. Bellinghan, G.R. Bernard, J.D. Chiche, C. Coopersmith, D.P. De Backer, C.J. French, S. Fujishima, H. Gerlach, J.L. Hidalgo, S.M. Hollenberg, A.E. Jones, D.R. Karnad, R.M. Kleinpell, Y. Koh, T.C. Lisboa, F.R. Machado, J.J. Marini, J.C. Marshall, J.E. Mazuski, L.A. McIntyre, A.S. McLean, S. Mehta, R.P. Moreno, J. Myburgh, P. Navalesi, O. Nishida, T.M. Osborn, A. Perner, C.M. Plunkett, M. Ranieri, C.A. Schorr, M.A. Seckel, C.W. Seymour, L. Shieh, K.A. Shukri, S.Q. Simpson, M. Singer, B.T. Thompson, S.R. Townsend, T. Van der Poll, J.L. Vincent, W.J. Wiersinga, J.L. Zimmerman, and R.P. Dellinger. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Critical Care Medicine 45: 486–552.
Vallet, B. 2003. Bench-to-bedside review: endothelial cell dysfunction in severe sepsis: a role in organ dysfunction. Critical Care: The Official Journal of the Critical Care Forum 7: 130–138.
Siragusa, M., and I. Fleming. 2016. The eNOS signalosome and its link to endothelial dysfunction. Pflügers Archiv : European Journal of Physiology 468: 1125–1137.
Kolluru, G.K., J.H. Siamwala, and S. Chatterjee. 2010. eNOS phosphorylation in health and disease. Biochimie 92: 1186–1198.
Hein, O.V., K. Misterek, J.P. Tessmann, V. van Dossow, M. Krimphove, and C. Spies. 2005. Time course of endothelial damage in septic shock: prediction of outcome. Critical Care: the Official Journal of the Critical Care Forum 9: R323–R330.
Duffy, M.J., B.A. Mullan, T.R. Craig, M. Shyamsundar, R.E. MacSweeney, G. Thompson, M. Stevenson, and D.F. McAuley. 2011. Impaired endothelium-dependent vasodilatation is a novel predictor of mortality in intensive care. Critical Care Medicine 39: 629–635.
Lamkanfi, M., and V.M. Dixit. 2014. Mechanisms and functions of inflammasomes. Cell 157: 1013–1022.
Latz, E., T.S. Xiao, and A. Stutz. 2013. Activation and regulation of the inflammasomes. Nature Reviews. Immunology 13: 397–411.
Danielski, L.G., A.D. Giustina, S. Bonfante, T. Barichello, and F. Petronilho. 2019. The NLRP3 inflammasome and its role in sepsis development. Inflammation 43 (1): 24–31.
Rittirsch, D., M.S. Huber-Lang, M.A. Flierl, and P.A. Ward. 2009. Immunodesign of experimental sepsis by cecal ligation and puncture. Nature Protocols 4: 31–36.
Kuhlencordt, P.J., E. Rosel, R.E. Gerszten, M. Morales-Ruiz, D. Dombkowski, W.J. Atkinson, F. Han, F. Preffer, A. Rosenzweig, W.C. Sessa, M.A. Gimbrone Jr., G. Ertl, and P.L. Huang. 2004. Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. American Journal of Physiology. Cell Physiology 286: C1195–C1202.
Cauwels, A. 2007. Nitric oxide in shock. Kidney International 72: 557–565.
Coletta, C., K. Módis, G. Oláh, A. Brunyánszki, D.S. Herzig, E.R. Sherwood, Z. Ungvári, and C. Szabo. 2014. Endothelial dysfunction is a potential contributor to multiple organ failure and mortality in aged mice subjected to septic shock: preclinical studies in a murine model of cecal ligation and puncture. Critical Care : the Official Journal of the Critical Care Forum 18: 511.
Tsai, H.J., M.H. Liao, C.C. Shih, S.M. Ka, C.M. Tsao, and C.C. Wu. 2018. Angiotensin-(1-7) attenuates organ injury and mortality in rats with polymicrobial sepsis. Critical Care: the Official Journal of the Critical Care Forum 22: 269.
Seemann, S., F. Zohles, and A. Lupp. 2017. Comprehensive comparison of three different animal models for systemic inflammation. Journal of Biomedical Science 24: 60.
Zong, J.Q., S.Y. Wang, P. Su, B.B. Sun, M.D. Guo, G.H. Diao, J. Wang, and H.H. Wang. 2019. TLR4/NF-kappaB p65 signaling pathway mediates protective effect of triptolide on endothelium in rats with endotoxemia. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica 44: 4912–4917.
Shi, X., S. Wang, H. Luan, D. Tuerhong, Y. Lin, J. Liang, Y. Xiong, L. Rui, and F. Wu. 2019. Clinopodium chinense attenuates palmitic acid-induced vascular endothelial inflammation and insulin resistance through TLR4-mediated NF-κB and MAPK pathways. The American Journal of Chinese Medicine 47: 97–117.
Vozza, A., G. Parisi, F. De Leonardis, F.M. Lasorsa, A. Castegna, D. Amorese, R. Marmo, V.M. Calcagnile, L. Palmieri, D. Ricquier, E. Paradies, P. Scarcia, F. Palmieri, F. Bouillaud, and G. Fiermonte. 2014. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proceedings of the National Academy of Sciences of the United States of America 111: 960–965.
Rahim, I., B. Djerdjouri, R.K. Sayed, M. Fernández-Ortiz, B. Fernández-Gil, A. Hidalgo-Gutiérrez, L.C. López, G. Escames, R.J. Reiter, and D. Acuña-Castroviejo. 2017. Melatonin administration to wild-type mice and nontreated NLRP3 mutant mice share similar inhibition of the inflammatory response during sepsis. Journal of pineal research 63.
Wu, Y., J. Ren, B. Zhou, C. Ding, J. Chen, G. Wang, G. Gu, X. Wu, S. Liu, D. Hu, and J. Li. 2015. Gene silencing of non-obese diabetic receptor family (NLRP3) protects against the sepsis-induced hyper-bile acidaemia in a rat model. Clinical and Experimental Immunology 179: 277–293.
Cao, Y., D. Fei, M. Chen, M. Sun, J. Xu, K. Kang, L. Jiang, and M. Zhao. 2015. Role of the nucleotide-binding domain-like receptor protein 3 inflammasome in acute kidney injury. The FEBS Journal 282: 3799–3807.
Luo, Y.P., L. Jiang, K. Kang, D.S. Fei, X.L. Meng, C.C. Nan, S.H. Pan, M.R. Zhao, and M.Y. Zhao. 2014. Hemin inhibits NLRP3 inflammasome activation in sepsis-induced acute lung injury, involving heme oxygenase-1. International Immunopharmacology 20: 24–32.
Xu, F., F. Wang, T. Wen, W. Sang, D. Wang, and N. Zeng. 2017. Inhibition of NLRP3 inflammasome: a new protective mechanism of cinnamaldehyde in endotoxin poisoning of mice. Immunopharmacology and Immunotoxicology 39: 296–304.
Bruder-Nascimento, T., N.S. Ferreira, C.Z. Zanotto, F. Ramalho, I.O. Pequeno, V.C. Olivon, K.B. Neves, R. Alves-Lopes, E. Campos, C.A. Silva, R. Fazan, D. Carlos, F.L. Mestriner, D. Prado, F.V. Pereira, T. Braga, J.P. Luiz, S.B. Cau, P.C. Elias, A.C. Moreira, N.O. Câmara, D.S. Zamboni, J.C. Alves-Filho, and R.C. Tostes. 2016. NLRP3 inflammasome mediates aldosterone-induced vascular damage. Circulation 134: 1866–1880.
Chauhan, S.D., G. Seggara, P.A. Vo, R.J. Macallister, A.J. Hobbs, and A. Ahluwalia. 2003. Protection against lipopolysaccharide-induced endothelial dysfunction in resistance and conduit vasculature of iNOS knockout mice. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology 17: 773–775.
Poon, B.Y., E. Raharjo, K.D. Patel, S. Tavener, and P. Kubes. 2003. Complexity of inducible nitric oxide synthase: cellular source determines benefit versus toxicity. Circulation 108: 1107–1112.
Cobb, J.P., R.S. Hotchkiss, P.E. Swanson, K. Chang, Y. Qiu, V.E. Laubach, I.E. Karl, and T.G. Buchman. 1999. Inducible nitric oxide synthase (iNOS) gene deficiency increases the mortality of sepsis in mice. Surgery 126: 438–442.
Fu, Q., J. Wu, X.Y. Zhou, M.H. Ji, Q.H. Mao, Q. Li, M.M. Zong, Z.Q. Zhou, and J.J. Yang. 2019. NLRP3/Caspase-1 pathway-induced pyroptosis mediated cognitive deficits in a mouse model of sepsis-associated encephalopathy. Inflammation 42: 306–318.
Dinarello, C.A. 2011. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 117: 3720–3732.
Funding
This work was supported by the National Natural Science Foundation of China (81270210) and (31400999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Luo, M., Meng, J., Yan, J. et al. Role of the Nucleotide-Binding Domain-Like Receptor Protein 3 Inflammasome in the Endothelial Dysfunction of Early Sepsis. Inflammation 43, 1561–1571 (2020). https://doi.org/10.1007/s10753-020-01232-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01232-x