Abstract
Reactive oxygen species (ROS) are continuously produced as byproducts of aerobic metabolism. Oxidative stress (OS) plays an important role in the occurrence of several neurodegenerative diseases as well as aging because of the accumulation of ROS. Gnaq is a member of G protein α subunits. It has been reported that the expression level of Gnaq in the mouse forebrain cortex was significantly decreased with age in our previous study; therefore, we supposed that Gnaq contributes to attenuate the OS. In this study, we generated a Gnaq-overexpression cell using gene recombinant technique and lentivirus transfection technique in a neuron-like PC12 cell, and investigated whether Gnaq had antioxidant effects in PC12 cells treated with H2O2. The viability of cells, concentration of ROS, Nrf2 nuclear translocation, expression of antioxidant enzymes, activation of NF-κB and apoptosis were compared between Gnaq-PC12 cells and Vector-PC12 cells. Results showed that, compared with Vector-PC12 cells, the antioxidative ability of Gnaq-PC12 cells was significantly improved, while the ROS level in Gnaq-PC12 cells was significantly decreased. Nrf2 nuclear translocation was up-regulated and NF-κB nuclear translocation was down-regulated in Gnaq-PC12 cells after H2O2 treatment. The results suggest that Gnaq plays a crucial role in neuroprotection in PC12 cells. A possible mechanism for this would be that the overexpressed Gnaq enhances the antioxidative effect mediated by Nrf2 signal pathway and inhibits the cellular damaging effect through NF-κB signal pathway.
Similar content being viewed by others
Introduction
Gnaq is α subunit of heterotrimeric G proteins that are operating as downstream of G protein-coupled receptors (GPCRs) and can activate a number of alternative signaling cascades inside cells (Kankanamge et al. 2019; Fredriksson et al. 2003). The most recognized signal pathway of Gnaq is that it activates phospholipase C β (PLC β) to hydrolyze the phosphatidylinositol 4,5-bisphosphate to release two potent second messengers: inositol 1,4,5 trisphosphate (IP3) and diacylglycerol (DAG). DAG provides a docking site within the inner leaflet of the plasma membrane for protein kinase C (PKC) (Hubbard et al. 2006). IP3 releases calcium from the smooth endoplasmic reticulum which also activates PKC. Subsequently, PKC activates MAPK/ERK or Nuclear factor-κB (NF-κB) leading to a series of survival or inflammatory responses. In our previous study (Jia et al. 2017), overexpressed Gnaq in SY5Y cells, a neuron-like cell line from human adrenal pheochromocytoma, exerted significant antioxidative effect by decreasing NF-κB level, which is contradictory to the classical Gnaq pathway. It was surmised that there might exist some other unknown regulatory mechanisms whereby Gnaq would exert the antioxidative effect. Another issue that has remained to be ascertained is whether Gnaq would exert a similar antioxidative effect in neuron-like cells derived from other species.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key transcription factor controlling many aspects of cell homoeostasis in response to oxidative, toxic insults and tumor (Yang et al. 2019; Sanghvi et al. 2019). Under physiological conditions, Keap1 keeps Nrf2 in the cytoplasm, allowing it to be ubiquitinated and degraded by proteasomes (Cuadrado et al. 2019). When cells are exposed to oxidative stress, a signal involving phosphorylation and/or redox modification in Keap1 blocks the enzymatic activity of the Keap1-Cul3-Rbx1 E3 ubiquitin ligase complex, leading to decreased ubiquitination and degradation of Nrf2. As a result, free Nrf2 translocates into the nucleus, where it activates the antioxidant response element (ARE) of many cytoprotective genes (Fão et al. 2019). In particular, Nrf2 mediates both basal and induced transcription of antioxidant proteins, which are responsible for the clearance of ROS, providing protection against the accumulation of toxic metabolites (Kobayashi et al. 2004). Among the most studied Nrf2-target genes are NAD(P)H dehydrogenase quinone 1 (NQO1), heme oxygenase-1 (HO-1), and glutathione-s-transferase (GST) (Thimmulappa et al. 2002; Kumar et al. 2018).
In this study, we used gene recombinant technique and lentivirus transfection technique to create a Gnaq-overexpression cell model by using PC12 cells, a rat-adrenal- pheochromocytoma-derived neuron-like cell line. Gnaq-PC12 and Vector- PC12 cells were treated with H2O2 to build an oxidative stress (OS) cell model. Then, we detected the viability of cells, concentration of ROS, Nrf2 nuclear translocation, expression of antioxidant enzymes, activation of NF-κB and apoptosis. The aim of our current study is to investigate the role of Gnaq in PC12 cells with H2O2-induced OS and, furthermore, to explore the underlying mechanism by which Gnaq can protect neurons from OS.
Materials and Methods
Cell Culture and Treatment
Rat pheochromocytoma (PC12) cells were obtained from Genechem Co. Ltd (Shanghai, China) and cultured in DMEM/F12 (HyClone, Shanghai, China) containing 10% FBS (Gibco, Australia) in an incubator with 5% CO2 at 37 °C. In all experiments, cells were treated with or without the indicated concentrations of H2O2 in the FBS-free medium for different durations.
Gnaq-PC12 and Vector-PC12 Cell Line Construction
The Gnaq-overexpression PC12 cell line (Gnaq- PC12) and control cell line (Vector- PC12) were constructed by Genechem Co. Ltd. (Shanghai, China). In brief, the whole codon sequence of Gnaq (NM_031036) was amplified by PCR with the following primers: forward 5′-CCAACTTTGTGCCAACCGGTCGCC ACCATGACTCTGGAGTCCATCATGG-3′, reverse 5′-AATGCCAACTCTGAG CTTGACCAGATTGTACTCCTTCAGG-3′. The PCR product was cloned into lentivirus vector Ubi-MCS-3FLAG-SV40-puromycin (GV341-Gnaq) between AgeI and NheI sites. The sequence integrity was verified by sequencing. The pseudotyped lentiviruses were produced by cotransfecting 293 T cells with GV341-Gnaq or GV341 together with 2 other packaging vectors: pHelper 1.0 (contain gag, pol and rev gene) and pHelper 2 (contain VSV-G gene). Full medium with puro was used to select the target cells. PCR and western blot analysis were used to detect the overexpressing efficiency of Gnaq gene.
Cell Viability Assay (Cell Counting kit-8)
Cells were seeded into 96-well plates for 12 h and subjected to different treatments. They were cultured for different time points according to the experimental design. After this,10 μl per well of CCK-8 Kit reagent (DOJINDO, Japan) was added and incubated for 2 h incubation at 37 °C. The absorbance of each well was read at 450 nm on a microplate reader. All experiments were independently repeated at least three times.
ROS Measurement
The dihydroethidium (DHE) method was used to determine the level of intracellular ROS. Gnaq-PC12 and Vector- PC12 cells were seeded in a 6-well plate at 2.5 × 105 cells/well. Twelve hours after seeding, cells were incubated with H2O2 (150 μM) for 4 h at 37 °C. After giving 5 μM DHE solution, the reaction was maintained for 60 min in the dark, and then the fluorescence was detected using Olympus microscope (IX73, Japan) and flow cytometry (Accuri C6, BD, USA).
Preparation of Whole Cell, Cytoplasmic and Nuclear Protein
For the whole cell protein extraction, cells were incubated with RIPA lysis buffer (Solarbio). The cell lysates were then centrifuged and the supernatant was collected and stored. The cytoplasmic and nuclear protein was extracted by using the Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime, Shanghai, China) according to the protocol provided by the manufacturer. The protein content was assayed using the BCA protein quantification kit (Applygen, Beijing, China).
Western Blot Analysis
Protein samples (30 μg) were resolved by SDS-PAGE (7.5%–15%) and transferred to PVDF membranes. Membranes were blocked with 5% skim milk for 3 h, and then were incubated at 4 °C overnight with diluted primary antibodies (All antibodies were purchased from Cell Signaling Technology, USA, and were diluted as 1:1000). Membranes were washed and incubated with HRP-linked secondary antibody (Zsbio, Beijing, China; 1:2000) for 1.5 h at room temperature. Finally, bands were visualized using an ECLplus Western Blotting Detection Reagents kit. Images were captured by AI600 system and gray levels were measured by ImageJ software (NIH, Bethesda, USA).
Apoptosis Assay
Hoechst 33,258 apoptotic assay kit was purchased from Beyotime Institte Biotechnology Co., Ltd. (Shanghai, China). Experiments were performed according to the manufacturer’s protocols. In brief, Vector-PC12 cells and Gnaq-PC12 cells were seeded in 6-well plates in a concentration of 5 × 105 cells/mL. Twelve hours after seeding, cells were treated with 150 μM H2O2 for 4 h, and control cells were treated with the same medium of PBS. Then, medium was aspirated and replaced by reaction buffers (cell stain buffer 1 mL, Hoechst33258 stain 5 μL for each well). After 30 min of staining in the dark, the reaction buffers were discarded and cells were washed with PBS. The fluorescence was detected using an Olympus microscope (IX73, Japan) at 450 nm wavelength.
Immunofluorescence
Cells were washed, fixed, permeabilized and blocked. After that, cells were incubated at 4 °C overnight with the primary antibodies. On the following day, cells were incubated with secondary antibodies and DAPI. Finally, the cell samples were imaged using an Olympus microscope (BX53, Japan).
Graphing and Statistical Analysis
Graphing analyses were performed using GraphPad Prism software (ver. 6.0; GraphPad Software Inc., San Diego, CA, USA). Data were represented as means ± standard error of the mean (SEM). Statistical analysis of differences between two groups was done using the independent-samples t test and one-way or two-way ANOVA (SPSS 16.0). P < 0.05 was considered as statistically significant in all analyses.
Results
Gnaq-Overexpressing PC12 And Control Cell Lines Were Successfully Constructed
To test our hypothesis, first, we obtained two qualified cell line (Gnaq-PC12 model cell line and Vector-PC12 control cell line) by gene recombinant engineering, lentiviral-transfection technique and puro-selection process. Next, we verified that Gnaq protein was successfully overexpressed in the Gnaq-PC12 model cell line by Western Blot (Fig. 1a) that was mainly expressed in the cytoplasm (Fig. 1b).
Gnaq-overexpressing PC12 and control cell lines were successfully constructed. a Total Gnaq proteins of Gnaq-PC12 model cell line and Vector-PC12 control cell line in the whole cells were measured by Western blot. Data were presented as means ± SEM of three independent experiments, **P < 0.01. b Gnaq protein localization was detected via immunofluorescence staining labeled by Alexa Fluor 488-conjugated secondary antibody (Green); nuclei were counterstained with DAPI (Blue). Scale bar, 20 μm
Gnaq Overexpression Significantly Improved Antioxidative Ability and Attenuated Apoptosis of PC12 Cells Treated by H2O2
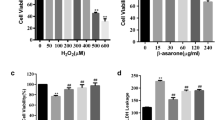
The oxidative stress model was established by H2O2 treatment in two transfected cell lines. In order to evaluate the range of effective concentrations and time, we assessed the cell viability by CCK8 experiment with H2O2 (50 to 400 μM) treatment for 0.5, 1, 2 and 4 h. As shown in Fig. 2, H2O2 had cytotoxic effects on both cell lines with the increase of its concentration and treatment time. When treated with 150 μM of H2O2 for 4 h, the cell viability was significantly inhibited (Fig. 2d). The ratio of surviving Vector-PC12 cells, in comparison with Gnaq-PC12 cells, decreased significantly (Fig. 2d). Based on this, we constructed the oxidative stress model with 150 μM of H2O2 for 4 h incubation in the subsequent experiments. Hoechst 33,258 apoptotic assay showed that more apoptosis was induced in Vector-PC12 cells than that in Gnaq-PC12 cells after being treated with 150 μM H2O2 for 4 h (Fig. 2e). Western blot analysis demonstrated that Bax was significantly lower and Bcl-2 was significantly higher in Gnaq-PC12 cells compared with Vector-PC12 cells after H2O2 stimulation (Fig. 2f).
Gnaq overexpression significantly improved antioxidative ability of PC12 cells treated by H2O2. Gnaq-PC12 and Vector-PC12 cells were treated with H2O2 (50 to 400 μM) for different time durations: a 0.5 h, b 1 h, c 2 h, d 4 h. Cell viability was determined by CCK8 assay. Data are presented as means ± SEM of three independent experiments. Control group was untreated cells. *P < 0.05 versus Vector-PC12 cells, **P < 0.01 versus Vector-PC12 cells, #P < 0.05 versus control group, ##P < 0.01 versus control group, ###P < 0.001 versus control group. e Cells treated with or without 150 μM H2O2 for 4 h were stained by Hoechst 33,258. Arrows indicate apoptotic cells. Scale bar, 20 μm. f Both cell lines were exposed to 150 μM of H2O2 for 4 h as needed; Bax and Bcl-2 were assessed by Western blot analysis. Data are presented as means ± SEM of three independent experiments. *P < 0.05 and **P < 0.01 versus Vector-PC12 cells
Gnaq Overexpression Significantly Decreased ROS Level in PC12 Cells Treated by H2O2
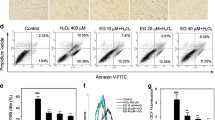
Intracellular ROS acts as an important second messenger in oxidative stress model. Hence, we determined the ROS production in Gnaq-PC12 and Vector-PC12 cells subjected to H2O2 (150 μM, 4 h) treatment. Dihydroethidium (DHE) labeled intracellular ROS was determined by fluorescence microscope. Results showed that ROS production was obviously decreased in Gnaq-PC12 cells versus the Vector-PC12 cells (Fig. 3a). Flow cytometry analysis also demonstrated that the level of ROS in Gnaq-PC12 cells was significantly lower than that in Vector-PC12 cells after H2O2 stimulation (Fig. 3b).
Gnaq overexpression significantly suppressed ROS production in PC12 cells treated by H2O2. a Representative images showed ROS positive cells of the different groups by DHE staining as described in experimental procedures. Scale bar, 100 μm. b Cells were detected by flow cytometry analysis. Data were presented as means ± SEM of three independent experiments. **P < 0.01 versus Vector-PC12 cells
Gnaq Overexpression Promoted Nuclear Translocation Of The Nrf2 And Enhanced Antioxidant Enzymes (HO-1, NQO-1 And GST) Expression
Considering that Nrf2 is a crucial endogenous antioxidative factor, we further investigated the role of Gnaq in Nrf2-related antioxidant pathways. Firstly, we found that the total Nrf2 protein between Gnaq-PC12 cells and Vector-PC12 cells with or without H2O2 stimulation was comparable (Fig. 4a); on the other hand, Western blot analysis showed that Gnaq-PC12 cells could significantly induce the translocation of Nrf2 from the cytoplasm to the cell nucleus with or without H2O2 (150 μM, 4 h) stimulation (Fig. 4b, c). Similarly, immunofluorescence analysis also showed that Gnaq overexpression in PC12 cells could promote nuclear translocation of the Nrf2 with or without H2O2 stimulation (Fig. 4d). We next examined the levels of antioxidant enzymes HO-1, NQO-1 and GST in Gnaq-PC12 cells and Vector-PC12 cells with or without H2O2 (150 μM, 4 h) stimulation. As shown in Fig. 4e, after stimulation with H2O2 (150 μM, 4 h), the HO-1, NQO-1 and GST protein expression levels in Gnaq-PC12 cells were significantly enhanced versus the Vector-PC12 cells.
Gnaq overexpression promoted Nrf2 nuclear translocation and enhanced expression of antioxidant enzymes (HO-1, NQO-1 and GST). (A-E) Cells were treated with or without H2O2 (150 μM) for 4 h. a The level of total Nrf2 in two cell lines. b Expression level of Nrf2 in nuclear fraction. c Expression level of Nrf2 in cytoplasmic fraction. d Nrf2 protein localization was detected via immunofluorescence staining which was labeled by Alexa Fluor 594-conjugated secondary antibody (Green), and nuclei were counterstained with DAPI (Blue). Scale bar, 20 μm. e Protein expression levels of HO-1, NQ-O1 and GST as measured by Western blot in different groups. Data are presented as means ± SEM in triplicate. **P < 0.01, ***P < 0.001 and ##P < 0.01 versus control group
Gnaq Overexpression Inhibited Activation of the NF-κB in H2O2-Treated PC12 Cells
NF-κB/p65 nuclear translocation promotes pro-inflammatory gene expression and plays an important role in oxidative stress. To explore the role of Gnaq in NF-κB/p65 nuclear translocation, Nuclear and Cytoplasmic Protein Extraction Kit and immunofluorescence analysis were used. Western blot analysis showed that Gnaq-PC12 cells could significantly decrease the translocation of NF-κB from the cytoplasm to the cell nucleus when stimulated by H2O2 (150 μM, 4 h) (Fig. 5a, b). The results were confirmed by immunofluorescence labeling (Fig. 5c).
Gnaq overexpression inhibited NF-κB activation in H2O2-treated PC12 cells. a, b Cells were treated with or without H2O2 (150 μM) for 4 h, and extracted proteins were measured via Western blot. a Expression level of NF-κB in the nuclear fraction. b NF-κB expression level in the cytoplasmic fraction. c NF-κB protein localization was detected by immunofluorescence labeled by Alexa Fluor 488-conjugated secondary antibody (Red); cell nuclei were counterstained with DAPI (Blue). Scale bar, 20 μm. Data were presented as means ± SEM in triplicate. *P < 0.05 and #P < 0.01 versus control group
Discussion
It has been estimated that almost 40% of all identified G protein-coupled receptors (GPCRs) rely upon the Gnαq family to stimulate inositol lipid signaling (Hubbard et al. 2006). These receptors communicate signals from a number of hormones, neurotransmitters, chemokines, and autocrine and paracrine factors. Gnaq is rich in brain tissue. Activation of Gnaq signaling enhances memory consolidation and slows down cognitive decline (Arey et al. 2018). It has been reported that endogenous Gnaq-coupled neuromodulator receptors activate protein kinase A (Chen et al. 2017). We previously reported that the expression level of Gnaq in SAMP8 mouse forebrain cortex was significantly decreased with age (Chen et al. 2010), alluding to the possibility that Gnaq expression may be closely associated with brain aging. Gnaq plays a potential role in antioxidation in SY5Y cells as reported previously by us (Jia et al. 2017). This study aimed to investigate whether Gnaq could perform the same antioxidative function in a different neuron-like cell line, namely PC12 cells, in the hope to decipher the underlying mechanism of the antioxidative property of Gnaq.
The adrenal pheochromocytoma (PC12) cell line was originally isolated from a tumor in the adrenal medulla of a rat in 1976 (Greene et al. 1976). This is a cell line widely used as a neuron-like cell model for studying neurotoxicity and neuroprotection. In our study, Gnaq protein was overexpressed in the PC12 cells by gene recombinant engineering, lentiviral-transfection technique and puro-selection process. We showed here that Gnaq was preferentially localized in the cytoplasm to execute its function (Fig. 1). We further established the OS cell model by treatment of PC12 cells with H2O2. As shown in Fig. 2, when treated with 150 μM of H2O2 for 4 h, the viability of Vector-PC12 cells was significantly decreased; however, overexpression of Gnaq significantly improved the antioxidative ability of PC12 cells. When taken together with the results of our previous study (Jia et al. 2017), we are confident that Gnaq performs important antioxidative role in different neuron-like cells. This provides a promising molecular target for prevention and therapy of oxidative damage-related diseases such as AD and PD.
It has been reported that Gnaq may be a potential biomarker and therapeutic target for PD diagnosis and treatment (Liu et al. 2019) and mouse with Gnaq knockout showed impairments in motor functions and spatial working memory (Frederick et al. 2012). Mouse which lacks the Gnaq gene selectively in neuronal and glial precursor cells showed somatotroph hypoplasia, dwarfism and anorexia (Wettschureck et al. 2005), and enhanced Gnaq level increased GnRH secretion in sheep hypothalamic neurons (Zhai et al. 2019). Gnaq in neurons of tree shrews and mouse hippocampus was reduced by chronic psychosocial stress, and this kind of reduction was prevented by treatment with the antidepressant clomipramine and tianeptine, respectively (Alfonso et al. 2004 and 2006). These findings implicated that Gnaq plays significant role in maintaining normal neural function, as well as in the pathogenesis and development of neurodegenerative diseases like AD and PD. Because there is limited evidence from AD and PD, the role and related mechanisms of Gnaq in these neurodegenerative diseases deserves to be explored. It is noteworthy that there is still lack of evidence on Gnaq expression in glial cells. Remarkably, we have found Gnaq was strongly expressed in BV2 cells (a microglial cell line, data were not shown). It would therefore be necessary in the future to elucidate Gnaq expression in different glial cell types especially in physiological or pathological conditions. This would provide a more holistic view of the roles of Gnaq in the nervous system.
Meanwhile, the present results have shown that Gnaq overexpression could upregulate Bcl-2 and downregulate Bax to reduce apoptosis in OS model. Additionally, the expression level of ROS in Gnaq-PC12 cells was significantly suppressed which was markedly lower than that in Vector-PC12 cells after H2O2 stimulation (Fig. 3). Keap1/Nrf2 is an important adaptive pathway in response to oxidative stress. Nrf2 drives transcription of a myriad of genes, which encode a multitude of proteins involved in diverse cellular functions, including protein and organelle homoeostasis (Kwak et al. 2003; Jain et al. 2010). Many studies have pointed this pathway as a target of dietary phytochemicals (Lee et al. 2014). In the present study, we showed that Gnaq overexpression could significantly promote translocation of Nrf2 in both untreated or H2O2-treated PC12 cells. Of note, Nrf2 translocation in H2O2-treated Gnaq-PC12 cells was higher in comparison with the Vector-PC12 cells. Coupled with the increased translocation of Nrf2, the expression of antioxidant enzymes including HO-1, NQO-1 and GST was significantly enhanced (Fig. 4). These results indicated that the protective role of Gnaq involves apoptosis-related and radical clearance-related signal pathways. A possible mechanism for this would be that the overexpressed Gnaq had activated Nrf2, and increased the expression of antioxidative enzymes which conceivably would attenuate any neuronal damage caused by OS.
Activated NF-κB enters the nucleus to induce transcription of a variety of genes that mediate diverse cellular processes such as immunity, inflammation, proliferation, apoptosis and cellular senescence (Vaughan et al. 2011). In order to respond effectively to acute inflammation, NF-κB also prompts an increase in mitochondrial activity and NADPH oxidase expression, which are the main sources of the endogenous free radicals (Manea et al. 2007; Mauro et al. 2011). The present results have shown that overexpression of Gnaq inhibited translocation of the NF-κB/p65 in OS cell models (Fig. 5). Consistent with our previous findings (Jia et al. 2017) that overexpression of Gnaq in human SY5Y cells down-regulated the level of NF-κB/p65, upregulation of Gnaq in rat PC12 cells likewise had resulted in inhibition of NF-κB signal pathway to perform its antioxidative effect.
From the above, we concluded that overexpressed Gnaq can perform its neuroprotective role by enhancing the antioxidative effect through Nrf2 signal pathway, and by inhibiting the cellular damaging effect mediated by NF-κB signal pathway. Meanwhile, we still need to consider the potential cross-talk or interaction between Nrf2 and NF-κB pathway. It has been documented that increased Nrf2 activity in lupus nephritis leads to the accumulation of glutathione (GSH), which effectively buffers free radicals and prevents the activation of NF-κB/p65, resulting in reduced deposition of extracellular matrix (Jiang et al. 2014). Study by Yu has shown that NF-κB/p65 assists in increasing the abundance of nuclear Keap1 levels (Yu et al. 2011). This means the inhibition of NF-κB is not directly from Gnaq, but is due to the activated Nrf2 which enhanced the expression of antioxidant enzymes. This would offer a rational explanation that overexpressed Gnaq would ultimately inhibit NF-κB as demonstrated in the present study, instead of activating NF-κB as in the classical Gnaq signal pathway.
It is now clear that robust NF-κB and Nrf2 activity is essential for maintaining coordinated cellular responses to relieve the stress status of the nerve cells or other tissues. Imbalance between Nrf2 and NF-κB pathways is associated with a significant number of diseases ranging from neurodegeneration, autoimmune disorders and cancer (Ben-Neriah et al. 2011; Jeong et al. 2017). Based on our study, Gnaq plays an important role to regulate the fine balance of cellular redox status and responses to stress and inflammation under both physiological and disease conditions; hence, Gnaq may serve as a potential therapeutic target in neurodegenerative diseases.
References
Alfonso, J., Frick, L. R., Silberman, D. M., Palumbo, M. L., Genaro, A. M., & Frasch, A. C. (2006). Regulation of hippocampal gene expression is conserved in two species subjected to different stressors and antidepressant treatments. Biological Psychiatry, 59, 244–251.
Alfonso, J., Pollevick, G. D., Van Der Hart, M. G., Flugge, G., Fuchs, E., & Frasch, A. C. (2004). Identification of genes regulated by chronic psychosocial stress and antidepressant treatment in the hippocampus. European Journal of Neuroscience, 19, 659–666.
Arey, R. N., Stein, G. M., Kaletsky, R., Kauffman, A., & Murphy, C. T. (2018). Activation of G alpha q signaling enhances memory consolidation and slows cognitive decline. Neuron, 98, 562–574.
Ben-Neriah, Y., & Karin, M. (2011). Inflammation meets cancer, with NF-kappaB as the matchmaker. Nature Immunology, 12, 715–723.
Chen, S. C., Lu, G., Chan, C. Y., Chen, Y., Wang, H., Yew, D. T., et al. (2010). Microarray profile of brain aging-related genes in the frontal cortex of SAMP8. Journal of Molecular Neuroscience, 41, 12–16.
Chen, Y., Granger, A. J., Tran, T., Saulnier, J. L., Kirkwood, A., & Sabatini, B. L. (2017). Endogenous galphaq-coupled neuromodulator receptors activate protein kinase A. Neuron, 96, 1070–1083.
Cuadrado, A., Rojo, A. I., Wells, G., Hayes, J. D., Cousin, S. P., Rumsey, W. L., et al. (2019). Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nature Review Drug Discovery, 18(4), 295–317.
Fão, L., Mota, S. I., & Rego, A. C. (2019). Shaping the Nrf2-ARE-related pathways in Alzheimer's and Parkinson's diseases. Ageing Research Review. https://doi.org/10.1016/j.arr.2019.100942.
Frederick, A. L., Saborido, T. P., & Stanwood, G. D. (2012). Neurobehavioral phenotyping of G(aq) knockout mice reveals impairments in motor functions and spatial working memory without changes in anxiety or behavioral despair. Frontiers in Behavioral Neuroscience. https://doi.org/10.3389/fnbeh.2012.00029.
Fredriksson, R., Lagerstrom, M. C., Lundin, L. G., & Schioth, H. B. (2003). The G-protein-coupled receptors in the human genome form five main families, phylogenetic analysis, paralogon groups, and fingerprints. Molecular Pharmacology, 63, 1256–1272.
Greene, L. A., & Tischler, A. S. (1976). Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proceedings of the National Academy of Sciences of the United States of America, 73, 2424–2428.
Hubbard, K. B., & Hepler, J. R. (2006). Cell signalling diversity of the Gqalpha family of heterotrimeric G proteins. Cellular Signalling, 18, 135–150.
Jain, A., Lamark, T., Sjottem, E., Larsen, K. B., Awuh, J. A., Overvatn, A., et al. (2010). p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of Biological Chemistry, 285, 22576–22591.
Jeong, H., Liu, Y., & Kim, H. S. (2017). Dried plum and chokeberry ameliorate d-galactose-induced aging in mice by regulation of Pl3k/Akt-mediated Nrf2 and Nf-kB pathways. Experimental Gerontology, 95, 16–25.
Jia, N., Li, G., Huang, P., Guo, J., Wei, L., Lu, D., et al. (2017). Protective role and related mechanism of Gnaq in neural cells damaged by oxidative stress. Acta Biochimica et Biophysica Sinica, 49, 428–434.
Jiang, T., Tian, F., Zheng, H., Whitman, S. A., Lin, Y., Zhang, Z., et al. (2014). Nrf2 suppresses lupus nephritis through inhibition of oxidative injury and the NF-kappaB-mediated inflammatory response. Kidney International, 85, 333–343.
Kankanamge, D., Tennakoon, M., Weerasinghe, A., Cedeno-Rosario, L., Chadee, D. N., & Karunarathne, A. (2019). G protein αq exerts expression level-dependent distinct signaling paradigms. Cellular Signalling, 58, 34–43.
Kobayashi, A., Ohta, T., & Yamamoto, M. (2004). Unique function of the Nrf2-Keap1 pathway in the inducible expression of antioxidant and detoxifying enzymes. Methods in Enzymology, 378, 273–286.
Kumar, M., & Sandhir, R. (2018). Neuroprotective effect of hydrogen sulfide in hyperhomocysteinemia is mediated through antioxidant action involving Nrf2. Neuromolecular Med, 20(4), 475–490.
Kwak, M. K., Wakabayashi, N., Greenlaw, J. L., Yamamoto, M., & Kensler, T. W. (2003). Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Molecular and Cellular Biology, 23, 8786–8794.
Lee, J., Jo, D. G., Park, D., Chung, H. Y., & Mattson, M. P. (2014). Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: Focus on the nervous system. Pharmacological Reviews, 66, 815–868.
Liu, X., Chen, J., Guan, T., Yao, H., Zhang, W., Guan, Z., et al. (2019). miRNAs and target genes in the blood as biomarkers for the early diagnosis of Parkinson’s disease. BMC Systems Biology. https://doi.org/10.1186/s12918-019-0680-4.
Manea, A., Manea, S. A., Gafencu, A. V., & Raicu, M. (2007). Regulation of NADPH oxidase subunit p22 (phox) by NF-kB in human aortic smooth muscle cells. Archives of Physiology and Biochemistry, 113, 163–172.
Mauro, C., Leow, S. C., Anso, E., Rocha, S., Thotakura, A. K., Tornatore, L., et al. (2011). NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nature Cell Biology, 13, 1272–1279.
Sanghvi, V. R., Leibold, J., Mina, M., Mohan, P., Berishaj, M., Li, Z., et al. (2019). The oncogenic action of NRF2 depends on de-glycation by fructosamine-3-kinase. Cell, 178(4), 807–819.
Thimmulappa, R. K., Mai, K. H., Srisuma, S., Kensler, T. W., Yamamoto, M., & Biswal, S. (2002). Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Research, 62, 5196–5203.
Vaughan, S., & Jat, P. S. (2011). Deciphering the role of nuclear factor-kappaB in cellular senescence. Aging, 3, 913–919.
Wettschureck, N., Moers, A., Wallenwein, B., Parlow, A. F., Maser-Gluth, C., & Offermanns, S. (2005). Loss of Gq/11 family G proteins in the nervous system causes pituitary somatotroph hypoplasia and dwarfism in mice. Molecular and Cellular Biology, 25, 1942–1948.
Yang, Y., Willis, T. L., Button, R. W., Strang, C. J., Fu, Y., Wen, X., et al. (2019). Cytoplasmic DAXX drives SQSTM1/p62 phase condensation to activate Nrf2-mediated stress response. Nature Communications, 10, 3759. https://doi.org/10.1038/s41467-019-11671-2.
Yu, M., Li, H., Liu, Q., Liu, F., Tang, L., Li, C., et al. (2011). Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cellular Signalling, 23, 883–892.
Zhai, M., Zhao, Z., Yang, M., Liang, Y., Liang, H., Xie, Y., et al. (2019). The effect of GNAQ methylation on GnRH secretion in sheep hypothalamic neurons. Journal of Cellular Biochemistry, 120, 19396–19405.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (Nos. 81260195; 81302362; 81460210), the Special-Combined-Funds between the Department of Science-technology of Yunnan Province and Kunming Medical University (2018FE001), and the Science and Technology Innovative Team Grant of Kunming Medical University (CXTD201905)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, X., Li, GP., Huang, P. et al. Gnaq Protects PC12 Cells from Oxidative Damage by Activation of Nrf2 and Inhibition of NF-kB. Neuromol Med 22, 401–410 (2020). https://doi.org/10.1007/s12017-020-08598-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-020-08598-z