Abstract
Background and purpose
Aflatoxins are one of the most important mycotoxins, which have been classified as Group I carcinogenic compounds by the International Agency for Research on Cancer. This investigation aimed to examine the effect of Propolis on inhibition of the Aspergillus parasiticus growth, aflatoxin production and expression of aflatoxin biosynthesis pathway genes.
Materials and methods
A standard strain of Aspergillus parasiticus (ATCC 15517) was used to perform antifungal susceptibility test, using a microdilution method in accordance with the CLSI M38-A2 guidelines. The aflatoxin concentrations in the control and treated media were determined by HPLC. Also, the quantitative changes in the level of nor-1, ver-1 and omtA genes expression in aflatoxin biosynthetic pathway were analyzed using Real-Time PCR method.
Results
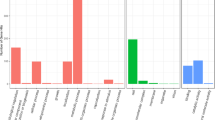
The results showed that the minimum inhibitory concentrations (MIC) of propolis was 100 µg/ml. The results showed that total levels of aflatoxin decreased from 386.1 ppm to 3.01 ppm at 50 µg/ml of propolis. In addition, quantitative real-time PCR analysis showed that the level of nor-1, ver-1 and omtA genes expression was significantly decreased after treatment with propolis extract.
Conclusions
The findings reveal that propolis extract, have a significant inhibitory effect on important genes for aflatoxin biosynthesis pathway in aflatoxin production.

Similar content being viewed by others
References
Hedayati MT, Omran SM, Soleymani A, Armaki MT. Aflatoxins in food products in Iran: a review of the literature. Jundishapur J Microbiol. 2016;9(7). https://doi.org/10.5812/jjm.33235.
Creppy EE. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol Lett. 2002;127(1–3):19–28. https://doi.org/10.1016/S0378-4274(01)00479-9.
Dehghan P, Zeyni F, Rezaei R, Jebali A, Kordbacheh P, Mahmoudi M. Detection of aflr gene and toxigenicity of Aspergillus flavus group isolated from patients with fungal sinusitis. 2008.
Ghanbari R, Molaee Aghaee E, Rezaie S, Jahed Khaniki G, Alimohammadi M, Soleimani M et al. The inhibitory effect of lactic acid bacteria on aflatoxin production and expression of aflR gene in Aspergillus parasiticus. J Food Saf. 2018;8(1):e12413. https://doi.org/10.1111/jfs.12413.
Mohseni R, Noorbakhsh F, Moazeni M, Nasrollahi Omran A, Rezaie S. Antitoxin characteristic of licorice extract: the inhibitory effect on aflatoxin production in Aspergillus parasiticus. J Food Saf. 2014;34(2):119–25. https://doi.org/10.1111/jfs.12104.
Khoshpey B, Farhud D, Zaini F. Aflatoxins in Iran: nature, hazards and carcinogenicity. Iran J Public Health. 2011;40(4):1.
Baydar T, Engin AB, Girgin G, Aydin S, Sahin G. Aflatoxin and ochratoxin in various types of commonly consumed retail ground samples in Ankara, Turkey. Ann Agric Environ Med. 2005;12(2):193–7.
Mardani M, Rezapour S, Rezapour P. Survey of aflatoxins in Kashkineh: A traditional Iranian food. Iran J Microbiol. 2011;3(3):147.
Pitt JI, Miller JD. A concise history of mycotoxin research. J Agric Food Chem 2017;65(33):7021–33. https://doi.org/10.1021/acs.jafc.6b04494.
Fouad AM, Ruan D, El-Senousey HK, Chen W, Jiang S, Zheng C. Harmful effects and control strategies of aflatoxin b1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry. Toxins. 2019;11(3):176. https://doi.org/10.3390/toxins11030176.
De Ruyck K, De Boevre M, Huybrechts I, De Saeger S. Dietary mycotoxins, co-exposure, and carcinogenesis in humans: short review. Mutat Res Rev Mutat Res. 2015;766:32–41. https://doi.org/10.1016/j.mrrev.2015.07.003.
IARC. Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. 1993;56. https://doi.org/10.1191/0960327103ht394oa.
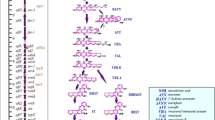
Yu J, Chang P-K, Ehrlich KC, Cary JW, Bhatnagar D, Cleveland TE et al. Clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol. 2004;70(3):1253–62. https://doi.org/10.1128/aem.70.3.1253-1262.2004.
Jahanshiri Z, Shams-Ghahfarokhi M, Allameh A, Razzaghi-Abyaneh M. Inhibitory effect of eugenol on aflatoxin B 1 production in Aspergillus parasiticus by downregulating the expression of major genes in the toxin biosynthetic pathway. World J Microbiol Biotechnol. 2015;31(7):1071–8. https://doi.org/10.1007/s11274-015-1857-7.
Yu J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins. 2012;4(11):1024–57. https://doi.org/10.3390/toxins4111024.
Bennett JW, Chang PK, Bhatnagar D. One gene to whole pathway: the role of norsolorinic acid in aflatoxin research. Adv Appl Microbiol. 1997;45:1–15. https://doi.org/10.1016/S0065-2164(08)70260-0.
Liang S-H, Wu T-S, Lee R, Chu FS, Linz JE. Analysis of mechanisms regulating expression of the ver-1 gene, involved in aflatoxin biosynthesis. Appl Environ Microbiol 1997;63(3):1058–65. https://doi.org/10.1128/AEM.63.3.1058-1065.1997.
Yabe K, Ando Y, Hashimoto J, Hamasaki T. Two distinct O-methyltransferases in aflatoxin biosynthesis. Appl Environ Microbiol. 1989;55(9):2172–7. https://doi.org/10.1128/AEM.55.9.2172-2177.1989.
Dana MA, Kordbacheh P, Ghazvini RD, Moazeni M, Nazemi L, Rezaie S. Inhibitory effect of vitamin C on Aspergillus parasiticus growth and aflatoxin gene expression. Curr Med Mycol. 2018;4(3):10. https://doi.org/10.18502/cmm.4.3.170.
Thippeswamy S, Mohana D, Abhishek R, Manjunath K. Inhibitory activity of plant extracts on aflatoxin B1 biosynthesis by Aspergillus flavus. J Agric Sci Technol. 2014;16(5):1123–32.
Schnürer J, Magnusson J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci Technol. 2005;16(1–3):70–8. https://doi.org/10.1016/j.tifs.2004.02.014.
Khan R, Islam B, Akram M, Shakil S, Ahmad AA, Ali SM et al. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules. 2009;14(2):586–97. https://doi.org/10.3390/molecules14020586.
Yan P-S, Song Y, Sakuno E, Nakajima H, Nakagawa H, Yabe K. Cyclo (L-leucyl-L-prolyl) produced by Achromobacter xylosoxidans inhibits aflatoxin production by Aspergillus parasiticus. Appl Environ Microbiol. 2004;70(12):7466–73. https://doi.org/10.1128/AEM.70.12.7466-7473.2004.
Rasooli I, Abyaneh MR. Inhibitory effects of Thyme oils on growth and aflatoxin production by Aspergillus parasiticus. Food Control. 2004;15(6):479–83. https://doi.org/10.1016/j.foodcont.2003.07.002.
Razzaghi-Abyaneh M, Shams-Ghahfarokhi M, Yoshinari T, Rezaee M-B, Jaimand K, Nagasawa H et al. Inhibitory effects of Satureja hortensis L. essential oil on growth and aflatoxin production by Aspergillus parasiticus. Int J Food Microbiol. 2008;123(3):228–33. https://doi.org/10.1016/j.ijfoodmicro.2008.02.003.
Münstedt K, Bogdanov S. Bee products and their potential use in modern medicine. J ApiProduct ApiMedical Sci. 2009;1(3):57–63. https://doi.org/10.3896/IBRA.4.01.3.01.
Zeighampour F, Mohammadi SM, Shams E, Naghavi NS. Antibacterial activity of propolis ethanol extract against antibiotic resistance bacteria isolated from burn wound infections. Zahedan J Res Med Sci. 2014;16(3):25–30.
Silva-Carvalho R, Baltazar F, Almeida-Aguiar C. Propolis: a complex natural product with a plethora of biological activities that can be explored for drug development. Evid Based Complement Alternat Med. 2015;2015. https://doi.org/10.1155/2015/206439.
Koç AN, Silici S, Kasap F, Hörmet-Öz HT, Mavus-Buldu H, Ercal BD. Antifungal activity of the honeybee products against Candida spp. and Trichosporon spp. J Med Food. 2011;14(1–2):128–34. https://doi.org/10.1089/jmf.2009.0296.
CLSI. Reference method for broth dilution antimicrobial susceptibility testing of filamentous fungi: approved standard M38-A2. 2008.
Pour SH, Mahmoudi S, Masoumi S, Rezaie S, Barac A, Ranjbaran M, et al. Aflatoxin M1 contamination level in Iranian milk and dairy products: a systematic review and meta-analysis. World Mycotoxin J. 2019:1–16. https://doi.org/10.3920/WMJ2019.2485.
Wang J-S, Shen X, He X, Zhu Y-R, Zhang B-C, Wang J-B et al. Protective alterations in phase 1 and 2 metabolism of aflatoxin B 1 by oltipraz in residents of Qidong, People’s Republic of China. J Natl Cancer Inst 1999;91(4):347–54. https://doi.org/10.1093/jnci/91.4.347.
Vaziri S, Dalvand M. Evaluation of antimicrobial effects of Zataria multiflora, Rosemarinus officinalis and Cuminum cyminum essential oils on the growth of Escherichia coli O157: H7. J Food Saf Hyg. 2018;4(3/4).
Vernozy-Rozand C, Ray‐Gueniot S, Ragot C, Bavai C, Mazuy C, Montet M et al. Prevalence of Escherichia coli O157: H7 in industrial minced beef. Lett Appl Microbiol. 2002;35(1):7–11.
Khodavaisy S, Rezaie S, Noorbakhsh F, Baghdadi E, Sharifynia S, Aala F. Effects of Pistacia atlantica subsp. kurdica on Growth and Aflatoxin Production by Aspergillus parasiticus. Jundishapur J Microbiol. 2016;9(7). https://doi.org/10.5812/jjm.35452.
Yahyaraeyat R, Khosravi A, Shahbazzadeh D, Khalaj V. The potential effects of Zataria multiflora Boiss essential oil on growth, aflatoxin production and transcription of aflatoxin biosynthesis pathway genes of toxigenic Aspergillus parasiticus. Braz J Microbiol. 2013;44(2):649–55. https://doi.org/10.1590/S1517-83822013000200045.
Shokri H, Khosravi AR, Yalfani R. Antifungal efficacy of propolis against fluconazole-resistant Candida glabrata isolates obtained from women with recurrent vulvovaginal candidiasis. Int J Gynecol Obstet. 2011;114(2):158–9. https://doi.org/10.1016/j.ijgo.2011.02.019.
Velazquez C, Navarro M, Acosta A, Angulo A, Dominguez Z, Robles R et al. Antibacterial and free-radical scavenging activities of Sonoran propolis. J Appl Microbiol. 2007;103(5):1747–56. https://doi.org/10.1111/j.1365-2672.2007.03409.x.
Agüero MB, Svetaz L, Sánchez M, Luna L, Lima B, López ML et al. Argentinean Andean propolis associated with the medicinal plant Larrea nitida Cav.(Zygophyllaceae). HPLC–MS and GC–MS characterization and antifungal activity. Food Chem Toxicol. 2011;49(9):1970–8. https://doi.org/10.1016/j.fct.2011.05.008.
Ownagh A, Ebrahimzadeh S, Adibhesam M. Comparative study on the effect of ethanol extract of propolis collected from West Azarbaijan apiaries against dermatophytes and non-dermatophytes fungi. J Urmia Univ Med Sci. 2010;21(3):206–14.
Soares MMSR, Cury AE. In vitro activity of antifungal and antiseptic agents against dermatophyte isolates from patients with tinea pedis. Braz J Microbiol. 2001;32(2):130–4. https://doi.org/10.1590/S1517-83822001000200012.
Jahanshiri Z, Shams-Ghahfarokhi M, Allameh A, Razzaghi-Abyaneh M. Effect of curcumin on Aspergillus parasiticus growth and expression of major genes involved in the early and late stages of aflatoxin biosynthesis. Iran J Public Health. 2012;41(6):72.
Yoshinari T, Akiyama T, Nakamura K, Kondo T, Takahashi Y, Muraoka Y et al. Dioctatin A is a strong inhibitor of aflatoxin production by Aspergillus parasiticus. Microbiology. 2007;153(8):2774–80. https://doi.org/10.1099/mic.0.2006/005629-0.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mahmoodzadeh Hosseini, H., Hamzeh Pour, S., Amani, J. et al. The effect of Propolis on inhibition of Aspergillus parasiticus growth, aflatoxin production and expression of aflatoxin biosynthesis pathway genes. J Environ Health Sci Engineer 18, 297–302 (2020). https://doi.org/10.1007/s40201-020-00467-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-020-00467-y