Abstract
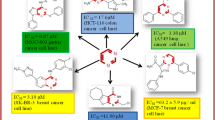
Synthesis of novel α-aminophosphonate hybrids 8a–f was accomplished by the reaction of 11-phenoxy-4-formylneocryptolepine 3, aminoalkylamino acridines 6a–f and diphenyl phosphite 7 in the presence of lithium perchlorate as Lewis acid catalyst in methanol. The starting aldehyde 3 was obtained by reaction of 11-chloroneocryptolpine 1 with 4-hydroxybenzaldehyde 2 in the presence of potassium carbonate in DMF, whereas 9-aminoalkylamino acridines 6a–f were synthesized by reaction of 9-chloroacridine 4 with appropriate diamines 5a–f. The structure of all synthesized hybrids was confirmed by spectroscopic methods and showed spectra in consistence with the expected structures. The antiproliferative activity of all synthesized hybrids was evaluated against HCT-116, MCF-7, HepG2 and A549 human cancer cell lines. The screened results proved that most of the reported compounds are potent, especially hybrid 8a, which displayed the highest activity against MCF-7, HepG2 and A549 cancer cell lines with IC50 8.2, 23.1 and 19.4 µM, respectively. This activity is significantly higher than the standard drug doxorubicin. Moreover, hybrid 8f showed most potent activity against HCT-116 cancer cell line with IC50 2.4 µM higher than the reference drug doxorubicin (IC50:10.90 µM).











Similar content being viewed by others
References
A.A.H. Sebeka, A.M.A. Osman, I.E.T. El-Sayed, M. El Bahanasawy, M.A. Tantawy, JAPS 7, 9 (2017)
A.A. El-Gokha, N.M. Boshta, M.K. Abo Hussein, I.E.T. El-Sayed, Chem Res Chin. Univ. 3, 373 (2017)
I.E.T. El-sayed, S. Elkhabiry, M. Świtalska, L. Wang, N. Wang, F. Xiu, I. Hayashi, T.A. Ngoc, S. Nagae, S. El-Ghlban, S. Shimoda, A.A. El-Gokha, J. Wietrzyk, T. Inokuchi, Molecules 22, 1954 (2017)
S.M. Emam, I.E.T. El Sayed, M.I. Ayad, H.M.R. Hathout, J. Mol. Struct. 1146, 600 (2017)
L. Larghi Enrique, A.B.J. Bracca, A.A. Arroyo Aguilar, D.A. Heredia, J.L. Pergomet, S.O. Simonetti, T.S. Kaufman, Curr. Top. Med. Chem. 15, 1683 (2015)
N. Wang, M. Świtalska, L. Wang, S. Elkhabiry, I. Hossain, I.E.T. El Sayed, J. Wietrzyk, T. Inokuchi, Molecules 24, 2121 (2019)
D. Gibson, Pharmacogenomics J. 2, 275 (2002)
A. Schmidt, M. Liu, Adv. Heterocycl. Chem. 115, 287 (2015)
F.A.A. Ibrahim, I.E.T. El Sayed, EJPMR 5, 59 (2018)
M. Kukowska, EUFEPS 109, 587 (2017)
A.A. El Gokha, A.A.S. Ahmed, N.A.M. Abdelwahed, I.E.T. El Sayed, Int. J. Pharm. Sci. Rev. Res. 36, 35 (2016)
M.A. Hamed, A.A. El Gokha, A.F.A. Ahmed, A. El Sayed, M. Sabry, R. Tarabee, A.El S. Abdel Megeed, I.E.T. El Sayed, Int. J. Pharm. Sci. Rev. Res. 34, 205 (2015)
P. Sreelakshmi, R.N. Maheshwara, S. Santhisudha, G. Mohan, N. Saichaithanya, M.S. Shaik, K. Peddanna, C. Nagaraju, S.R. Cirandur, Med. Chem. Res. 28, 528 (2019)
A.B. Danne, S.V. Akolkar, T.R. Deshmukh, M.M. Siddiqui, B.B. Shingate, J. Iran. Chem. Soc. 16, 953 (2019)
H.A.L. El-Boraey, A.A. El-Gokha, I.E.T. El Sayed, Med. Chem. Res. 24, 2142 (2015)
K. Nurgali, R. Jagoe Thomas, R. Abalo, Front. Pharmacol. (2018). https://doi.org/10.3389/fphar.2018.002457
B. Mansoori, A. Mohammadi, S. Davudian, S. Shirjang, B. Baradaran, Adv. Pharm. Bull. 7, 339 (2017)
G. Housman, S. Byler, S. Heerboth, K. Lapinska, M. Longacre, N. Snyder, S. Sarkar, Cancers 6, 1769 (2014)
K.P. Rakesh, S.-M. Wang, J. Leng, L. Ravindar, A.M. Asiri, H.M. Marwani, H.L. Qin, Anti-cancer Agent Med. 18, 488 (2018)
E. Balentová, J. Imrich, J. Bernáat, L. Suchá, M. Vilková, P. Kristian, K. Pihlaja, K.D. Klika, N. Pröanayová, J. Heterocycl. Chem. 43, 645 (2006)
A.S. Hassan, M.F. Mady, H.M. Awad, T.S. Hafez, Chin. Chem. Lett. 28, 388 (2017)
A.N. Emam, S.A. Loutfy, A.A. Mostafa, H.M. Awad, M.B. Mohamed, RSC Adv. 7, 23502 (2017)
E.M. Flefel, W.A. El-Sayed, A.M. Mohamed, W.I. El-Sofany, H.M. Awad, Molecules 22, 1 (2017)
R.S. Macomber, A Complete Introduction to Modern NMR Spectroscopy (Wiley, New York, 1998)
L. Xie, Y. Xu, F. Wang, J. Liu, X. Qian, J. Cuia, Bioorg. Med. Chem. 17, 804 (2009)
Funding
We would like to acknowledge financial support by Menoufia University throughout the Project Number Ib-C2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmed, A.A.S., Awad, H.M., El-Sayed, I.ET. et al. Synthesis and antiproliferative activity of new hybrids bearing neocryptolepine, acridine and α-aminophosphonate scaffolds. J IRAN CHEM SOC 17, 1211–1221 (2020). https://doi.org/10.1007/s13738-019-01849-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-019-01849-2