Abstract
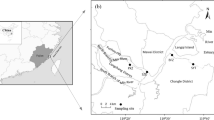
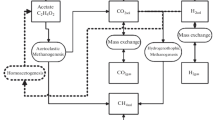
Methane production usually increases from the acidic sphagnum-dominated ombrotrophic peatlands to minerotrophic ones with more neutral pH and higher coverage of vascular plants. Along this ombrotrophic–minerotrophic gradient, pH, microbial communities, and properties of dissolved organic matter in porewater all vary greatly. The hydrographic change resulted from permafrost thaw and projected global warming can potentially connect the minerotrophic and ombrotrophic sites via porewater and turn acidic bogs to minerotrophic fens. It is thus very important to investigate how the anaerobic carbon degradation processes respond to changes in fundamental factors like pH, temperature, properties of dissolved organic matter, and microbial communities resulted from such hydrographic change. In this study, one ombrotrophic (pH = 3.9) and one minerotrophic peatland site were sampled in Fairbanks, Alaska in Sep 2017 and a 42-day-period anaerobic laboratory incubation was conducted to study the changes in anaerobic carbon degradation processes including primary and secondary fermentation, methanogenesis, and acetogenesis when pH, temperature, and porewater were manipulated individually and a combination of two or three of these factors. The results suggested lowering pH would inhibit many anaerobic carbon degradation processes in the minerotrophic peatland except primary fermentation. Elevating pH in the ombrotrophic site did not stimulate its methanogen community, but primary fermentation responded better with increasing pH than with increasing temperature alone. Replacing the porewater in the minerotrophic site with that from the ombrotrophic site with high aromaticity did not inhibit methanogenesis but potentially brought in highly efficient primary fermenters. Acetoclastic methanogenesis, acetogenesis, and syntrophy only exist in the minerotrophic site but not at the ombrotrophic one. Porewater from the minerotrophic site could potentially introduce acetoclastic methanogens and syntrophs to the ombrotrophic site but would not make them active unless both pH and temperature were increased. When ground water connects ombrotrophic and minerotrophic peatlands due to thawing of permafrost, secondary fermenters and acetoclastic methanogens could be introduced to acidic bogs and cooperate efficiently to degrade the stored carbon in ombrotrophic peatlands especially under elevated temperature conditions.







Similar content being viewed by others
References
Aerts R, Verhoeven JT, Whigham DF (1999) Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology 80(7):2170–2181
Avery GB, Shannon RD, White JR, Martens CS, Alperin MJ (2003) Controls on methane production in a tidal freshwater estuary and a peatland: methane production via acetate fermentation and CO2 reduction. Biogeochemistry 62(1):19–37
Balk M, Weijma J, Stams AJ (2002) Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int J Syst Evol Microbiol 52(4):1361–1368
Bellisario LM, Bubier JL, Moore TR, Chanton JP (1999) Controls on CH4 emissions from a northern peatland. Global Biogeochem Cycles 13(1):81–91
Bergman I, Lundberg P, Nilsson M (1999) Microbial carbon mineralisation in an acid surface peat: effects of environmental factors in laboratory incubations. Soil Biol Biochem 31(13):1867–1877
Blodau C, Roehm CL, Moore TR (2002) Iron, sulfur, and dissolved carbon dynamics in a northern peatland. Archiv für Hydrobiologie 1:561–583
Boone DR (1982) Terminal reactions in the anaerobic digestion of animal waste. Appl Environ Microbiol 43(1):57–64
Bräuer SL, Cadillo-Quiroz H, Yashiro E, Yavitt JB, Zinder SH (2006) Isolation of a novel acidiphilic methanogen from an acidic peat bog. Nature 442(7099):192
Bräuer SL, Cadillo-Quiroz H, Ward RJ, Yavitt JB, Zinder SH (2011) Methanoregula boonei gen. nov., sp. nov. an acidiphilic methanogen isolated from an acidic peat bog. Int J Syst Evol Microbiol 61(1):45–52
Braun K, Gottschalk G (1981) Effect of molecular hydrogen and carbon dioxide on chemo-organotrophic growth of Acetobacterium woodii and Clostridium aceticum. Arch Microbiol 128(3):294–298
Breznak JA, Blum JS (1991) Mixotrophy in the termite gut acetogen, Sporomusa termitida. Arch Microbiol 156(2):105–110
Bridgham SD, Updegraff K, Pastor J (1998) Carbon, nitrogen, and phosphorus mineralization in northern wetlands. Ecology 79(5):1545–1561
Bridgham SD, Pastor J, Updegraff K, Malterer TJ, Johnson K, Harth C, Chen J (1999) Ecosystem control over temperature and energy flux in northern peatlands. Ecol Appl 9(4):1345–1358
Bridgham SD, Cadillo-Quiroz H, Keller JK, Zhuang Q (2013) Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Change Biol 19(5):1325–1346
Bubier JL (1995) The relationship of vegetation to methane emission and hydrochemical gradients in northern peatlands. J Ecol 1:403–420
Chauhan A, Ogram A (2006) Phylogeny of acetate-utilizing microorganisms in soils along a nutrient gradient in the Florida Everglades. Appl Environ Microbiol 72(10):6837–6840
Cheema S, Zeyer J, Henneberger R (2015) Methanotrophic and methanogenic communities in Swiss alpine fens dominated by Carex rostrata and Eriophorum angustifolium. Appl Environ Microbiol 81(17):5832–5844
Chen S, Liu X, Dong X (2005) Syntrophobacter sulfatireducens sp. nov., a novel syntrophic, propionate-oxidizing bacterium isolated from UASB reactors. Int J Syst Evol Microbiol 55(3):1319–1324
Christensen TR, Ekberg A, Ström L, Mastepanov M, Panikov N, Öquist M, Svensson BH, Nykänen H, Martikainen PJ, Oskarsson H (2003) Factors controlling large scale variations in methane emissions from wetlands. Geophys Res Lett 30(7):10
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C (2013) Climate change 2013: the physical science basis. In: Tignor K, Allen M, Boschung SK, Nauels J, Xia A, Bex Y, Midgley VPM (eds) Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change
Clark JM, Chapman PJ, Adamson JK, Lane SN (2005) Influence of drought-induced acidification on the mobility of dissolved organic carbon in peat soils. Glob Change Biol 11(5):791–809
Clymo RS (1987) The ecology of peatlands. Sci Prog (1933) 1:593–614
Cokgor EU, Oktay S, Tas DO, Zengin GE, Orhon D (2009) Influence of pH and temperature on soluble substrate generation with primary sludge fermentation. Biores Technol 100(1):380–386
Conrad R (1999) Contribution of hydrogen to methane production and control of hydrogen concentrations in methanogenic soils and sediments (review). FEMS Microbiol Ecol 28:193–202
Conrad R, Bak F, Seitz HJ, Thebrath B, Mayer HP, Schütz H (1989) Hydrogen turnover by psychrotrophic homoacetogenic and mesophilic methanogenic bacteria in anoxic paddy soil and lake sediment. FEMS Microbiol Ecol 5(5):285–293
Dannenberg S, Wudler J, Conrad R (1997) Agitation of anoxic paddy soil slurries affects the performance of the methanogenic microbial community. FEMS Microbiol Ecol 22(3):257–263
De Bok FA, Harmsen HJ, Plugge CM, de Vries MC, Akkermans AD, de Vos WM, Stams AJ (2005) The first true obligately syntrophic propionate-oxidizing bacterium, Pelotomaculum schinkii sp. nov., co-cultured with Methanospirillum hungatei, and emended description of the genus Pelotomaculum. Int J Syst Evol Microbiol 55(4):1697–1703
Denman SE, Tomkins NW, McSweeney CS (2007) Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol Ecol 62(3):313–322
Dettling MD, Yavitt JB, Cadillo-Quiroz H, Sun C, Zinder SH (2007) Soil–methanogen interactions in two peatlands (bog, fen) in Central New York State. Geomicrobiol J 24(3–4):247–259
Dise NB, Gorham E, Verry ES (1993) Environmental factors controlling methane emissions from peatlands in northern Minnesota. J Geophys Res Atmos 98(D6):10583–10594
Duddleston KN, Kinney MA, Kiene RP, Hines ME (2002) Anaerobic microbial biogeochemistry in a northern bog: acetate as a dominant metabolic end product. Global Biogeochem Cycles 16(4):11–21
Falz KZ, Holliger C, Grosskopf R, Liesack W, Nozhevnikova AN, Müller B, Wehrli B, Hahn D (1999) Vertical distribution of methanogens in the anoxic sediment of Rotsee (Switzerland). Appl Environ Microbiol 65(6):2402–2408
Fey A, Conrad R (2000) Effect of temperature on carbon and electron flow and on the archaeal community in methanogenic rice field soil. Appl Environ Microbiol 66(11):4790–4797
Forster P, Ramaswamy V, Artaxo P, Berntsen T, Betts R, Fahey DW, Haywood J, Lean J, Lowe DC, Myhre G, Nganga J (2007) Changes in atmospheric constituents and in radiative forcing. Chapter 2. In: Climate Change 2007. The physical science basis
Frolking S, Crill P (1994) Climate controls on temporal variability of methane flux from a poor fen in southeastern New Hampshire: Measurement and modeling. Global Biogeochem Cycles 8(4):385–397
Fung I, John J, Lerner J, Matthews E, Prather M, Steele LP, Fraser PJ (1991) Three-dimensional model synthesis of the global methane cycle. J Geophys Res Atmos 96(D7):13033–13065
Fynn G, Syafila M (1990) Hydrogen regulation of acetogenesis from glucose by freely suspended and immobilised acidogenic cells in continuous culture. Biotech Lett 12(8):621–626
Galand P, Fritze H, Conrad R, Yrjälä K (2005) Pathways for methanogenesis and diversity of methanogenic archaea in three boreal peatland ecosystems. Appl Environ Microbiol 71(4):2195–2198
Galand P, Yrjälä K, Conrad R (2010) Stable carbon isotope fractionation during methanogenesis in three boreal peatland ecosystems. Biogeosciences 7:3893–3900
Glaser PH, Janssens JA, Siegel DI (1990) The response of vegetation to chemical and hydrological gradients in the Lost River peatland, northern Minnesota. J Ecol 3:1021–1048
Goodwin ST, Zeikus JG (1987a) Ecophysiological adaptations of anaerobic bacteria to low pH: analysis of anaerobic digestion in acidic bog sediments. Appl Environ Microbiol 53(1):57–64
Goodwin ST, Zeikus JG (1987b) Physiological adaptations of anaerobic bacteria to low pH: metabolic control of proton motive force in Sarcina ventriculi. J Bacteriol 169(5):2150–2157
Gorham E, Bayley SE, Schindler DW (1984) Ecological effects of acid deposition upon peatlands: a neglected field in" acid-rain" research. Can J Fish Aquat Sci 41(8):1256–1268
Gorham E, Janssens JA (2005) The distribution and accumulation of chemical elements in five peat cores from the mid-continent to the eastern coast of North America. Wetlands 25(2):259–278
Grybos M, Davranche M, Gruau G, Petitjean P, Pédrot M (2009) Increasing pH drives organic matter solubilization from wetland soils under reducing conditions. Geoderma 154(1–2):13–19
Harmsen HJ, Van Kuijk BL, Plugge CM, Akkermans AD, De Vos WM, Stams AJ (1998) Syntrophobacter fumaroxidans sp. nov., a syntrophic propionate-degrading sulfate-reducing bacterium. Int J Syst Evol Microbiol 48(4):1383–1387
Harper SR, Pohland FG (1986) Recent developments in hydrogen management during anaerobic biological wastewater treatment. Biotechnol Bioeng 28(4):585–602
Hattori S (2008) Syntrophic acetate-oxidizing microbes in methanogenic environments. Microbes and Environments 23(2):118–127
Hines ME, Duddleston KN, Rooney-Varga JN, Fields D, Chanton JP (2008) Uncoupling of acetate degradation from methane formation in Alaskan wetlands: connections to vegetation distribution. Global Biogeochem Cycles 22(2):10
Hoehler TM, Albert DB, Alperin MJ, Martens CS (1999) Acetogenesis from CO2 in an anoxic marine sediment. Limnol Oceanogr 44(3):662–667
Hori T, Noll M, Igarashi Y, Friedrich MW, Conrad R (2007) Identification of acetate-assimilating microorganisms under methanogenic conditions in anoxic rice field soil by comparative stable isotope probing of RNA. Appl Environ Microbiol 73(1):101–109
Horn MA, Matthies C, Küsel K, Schramm A, Drake HL (2003) Hydrogenotrophic methanogenesis by moderately acid-tolerant methanogens of a methane-emitting acidic peat. Appl Environ Microbiol 69(1):74–83
Imachi H, Sakai S, Ohashi A, Harada H, Hanada S, Kamagata Y, Sekiguchi Y (2007) Pelotomaculum propionicicum sp. nov., an anaerobic, mesophilic, obligately syntrophic, propionate-oxidizing bacterium. Int J Syst Evol Microbiol 57(7):1487–1492
IPCC, 2013, Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex B, Midgley BM (2013) Climate change 2013: the physical science basis. In: Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change
Jobbágy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10(2):423–436
Johnston CE, Ewing SA, Harden JW, Varner RK, Wickland KP, Koch JC, Fuller CC, Manies K, Jorgenson MT (2014) Effect of permafrost thaw on CO2 and CH4 exchange in a western Alaska peatland chronosequence. Environ Res Lett 9(8):085004
Kamagata Y, Mikami E (1989) Diversity of acetotrophic methanogens in anaerobic digestion. Recent Adv Microb Ecol 3:459–464
Keller JK, Bridgham SD (2007) Pathways of anaerobic carbon cycling across an ombrotrophic-minerotrophic peatland gradient. Limnol Oceanogr 52(1):96–107
Koch JM (2007) Alcoa’s mining and restoration process in south Western Australia. Restor Ecol 15:S11–S16
Kooijman AM, Bakker C (1993) Causes of the replacement of Scorpidium scorpioides by Calliergonella cuspidata in eutrophicated rich fens. 2. Experimental studies. Lindbergia 18(3):123–130
Kotsyurbenko OR, Glagolev MV, Nozhevnikova AN, Conrad R (2001) Competition between homoacetogenic bacteria and methanogenic archaea for hydrogen at low temperature. FEMS Microbiol Ecol 38(2–3):153–159
Kotsyurbenko OR, Chin KJ, Glagolev MV, Stubner S, Simankova MV, Nozhevnikova AN, Conrad R (2004) Acetoclastic and hydrogenotrophic methane production and methanogenic populations in an acidic West-Siberian peat bog. Environ Microbiol 6(11):1159–1173
Kotsyurbenko OR, Friedrich MW, Simankova MV, Nozhevnikova AN, Golyshin PN, Timmis KN, Conrad R (2007) Shift from acetoclastic to H2-dependent methanogenesis in a West Siberian peat bog at low pH values and isolation of an acidophilic Methanobacterium strain. Appl Environ Microbiol 73(7):2344–2348
Lamentowicz M, Lamentowicz Ł, van der Knaap WO, Gąbka M, Mitchell EA (2010) Contrasting species—environment relationships in communities of testate amoebae, bryophytes and vascular plants along the Fen-Bog gradient. Microb Ecol 59(3):499–510
Lamentowicz M, Bragazza L, Buttler A, Jassey VE, Mitchell EA (2013) Seasonal patterns of testate amoeba diversity, community structure and species–environment relationships in four Sphagnum-dominated peatlands along a 1300 m altitudinal gradient in Switzerland. Soil Biol Biochem 67:1–11
Lee MJ, Zinder SH (1988) Isolation and characterization of a thermophilic bacterium which oxidizes acetate in syntrophic association with a methanogen and which grows acetogenically on H2-CO2. Appl Environ Microbiol 54(1):124–129
Lelieveld JO, Crutzen PJ, Dentener FJ (1998) Changing concentration, lifetime and climate forcing of atmospheric methane. Tellus B 50(2):128–150
Li J, Ban Q, Zhang L, Jha AK (2012) Syntrophic propionate degradation in anaerobic digestion: a review. Int J Agric Biol 14(5):843–850
Maltby E, Immirzi P (1993) Carbon dynamics in peatlands and other wetland soils regional and global perspectives. Chemosphere 27(6):999–1023
McCalley CK, Woodcroft BJ, Hodgkins SB, Wehr RA, Kim EH, Mondav R, Crill PM, Chanton JP, Rich VI, Tyson GW, Saleska SR (2014) Methane dynamics regulated by microbial community response to permafrost thaw. Nature 514(7523):478
McQueen DJ (1990) OPINION Manipulating lake community structure: where do we go from here? Freshw Biol 23(3):613–620
Minderlein S, Blodau C (2010) Humic-rich peat extracts inhibit sulfate reduction, methanogenesis, and anaerobic respiration but not acetogenesis in peat soils of a temperate bog. Soil Biol Biochem 42(12):2078–2086
Mitchell EA, Buttler A, Grosvernier P, Rydin H, Albinsson C, Greenup AL, Heijmans MM, Hoosbeek MR, Saarinen T (2000) Relationships among testate amoebae (Protozoa), vegetation and water chemistry in five Sphagnum-dominated peatlands in Europe. The New Phytologist 145(1):95–106
Mitsch WJ, Gosselink JG (2000) The value of wetlands: importance of scale and landscape setting. Ecol Econ 35(1):25–33
Moore TR, Dalva M (1993) The influence of temperature and water table position on carbon dioxide and methane emissions from laboratory columns of peatland soils. J Soil Sci 44(4):651–664
Nüsslein B, Chin KJ, Eckert W, Conrad R (2001) Evidence for anaerobic syntrophic acetate oxidation during methane production in the profundal sediment of subtropical Lake Kinneret (Israel). Environ Microbiol 3(7):460–470
Phelps TJ, Zeikus JG (1984) Influence of pH on terminal carbon metabolism in anoxic sediments from a mildly acidic lake. Appl Environ Microbiol 48(6):1088–1095
Poulin BA, Ryan JN, Tate MT, Krabbenhoft DP, Hines ME, Barkay T, Schaefer J, Aiken GR (2019) Geochemical factors controlling dissolved elemental mercury and methylmercury formation in Alaskan Wetlands of varying trophic status. Environmental science and technology
Reddy KR, DeLaune RD (2008) Biogeochemistry of wetlands: science and applications. CRC Press, London
Rooney-Varga JN, Giewat MW, Duddleston KN, Chanton JP, Hines ME (2007) Links between archaeal community structure, vegetation type and methanogenic pathway in Alaskan peatlands. FEMS Microbiol Ecol 60(2):240–251
Rothfuss F, Conrad R (1993) Thermodynamics of methanogenic intermediary metabolism in littoral sediment of Lake Constance. FEMS Microbiol Ecol 12(4):265–276
Schink B (1994) Diversity, ecology, and isolation of acetogenic bacteria. In: Drake HL (eds) Acetogenesis. Chapman & hall microbiology series (Physiology / Ecology / Molecular Biology / Biotechnology). Springer, Boston, MA, pp 197–235
Schink B, Stams AJ (2013) Syntrophism among prokaryotes. Springer, Berlin, pp 471–493
Schwarz JI, Lueders T, Eckert W, Conrad R (2007) Identification of acetate-utilizing Bacteria and Archaea in methanogenic profundal sediments of Lake Kinneret (Israel) by stable isotope probing of rRNA. Environ Microbiol 9(1):223–237
Segers R (1998) Methane production and methane consumption: a review of processes underlying wetland methane fluxes. Biogeochemistry 41(1):23–51
Shannon RD, White JR (1996) The effects of spatial and temporal variations in acetate and sulfate on methane cycling in two Michigan peatlands. Limnol Oceanogr 41(3):435–443
Sieber JR, Sims DR, Han C, Kim E, Lykidis A, Lapidus AL, McDonnald E, Rohlin L, Culley DE, Gunsalus R, McInerney MJ (2010) The genome of Syntrophomonas wolfei: new insights into syntrophic metabolism and biohydrogen production. Environ Microbiol 12(8):2289–2301
Treat CC, Wollheim WM, Varner RK, Grandy AS, Talbot J, Frolking S (2014) Temperature and peat type control CO 2 and CH 4 production in Alaskan permafrost peats. Glob Change Biol 20(8):2674–2686
Treat CC, Natali SM, Ernakovich J, Iversen CM, Lupascu M, McGuire AD, Norby RJ, Roy Chowdhury T, Richter A, Šantrůčková H, Schädel C (2015) A pan-Arctic synthesis of CH4 and CO2 production form anoxic soil incubations. Glob Change Biol 21(7):2787–2803
Updegraff K, Bridgham SD, Pastor J, Weishampel P, Harth C (2001) Response of CO2 and CH4 emissions from peatlands to warming and water table manipulation. Ecol Appl 11(2):311–326
Urban NR, Eisenreich SJ, Gorham E (1987) Proton cycling in bogs: Geographic variation in Northeastern North America. In: Hutchinson TC, Meema KM (eds) Effects of atmospheric pollutants on forests, wetlands and agricultural ecosystems. NATO ASI series (Series G: Ecological Sciences), vol 16. Springer, Berlin, Heidelberg, pp 577–598
Valentine DW, Holland EA, Schimel DS (1994) Ecosystem and physiological controls over methane production in northern wetlands. J Geophys Res Atmos 99(D1):1563–1571
Van Lier JB, Grolle KC, Frijters CT, Stams AJ, Lettinga G (1993) Effects of acetate, propionate, and butyrate on the thermophilic anaerobic degradation of propionate by methanogenic sludge and defined cultures. Appl Environ Microbiol 59(4):1003–1011
Vile MA, Bridgham SD, Wieder RK (2003a) Response of anaerobic carbon mineralization rates to sulfate amendments in a boreal peatland. Ecol Appl 13(3):720–734
Vile MA, Bridgham SD, Wieder RK, Novák M (2003b) Atmospheric sulfur deposition alters pathways of gaseous carbon production in peatlands. Global Biogeochem Cycles 17(2):1058
Wang Q, Kuninobu M, Ogawa HI, Kato Y (1999) Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenerg 16(6):407–416
Williams CJ, Yavitt JB, Wieder RK, Cleavitt NL (1998) Cupric oxide oxidation products of northern peat and peat-forming plants. Can J Bot 76(1):51–62
Wuebbles DJ, Hayhoe K (2002) Atmospheric methane and global change. Earth Sci Rev 57(3–4):177–210
Yavitt JB, Seidman-Zager M (2006) Methanogenic conditions in northern peat soils. Geomicrobiol J 23(2):119–127
Yavitt JB, Williams CJ, Wieder RK (2000) Controls on microbial production of methane and carbon dioxide in three Sphagnum-dominated peatland ecosystems as revealed by a reciprocal field peat transplant experiment. Geomicrobiol J 17(1):61–88
Ye R, Jin Q, Bohannan B, Keller JK, McAllister SA, Bridgham SD (2012) pH controls over anaerobic carbon mineralization, the efficiency of methane production, and methanogenic pathways in peatlands across an ombrotrophic–minerotrophic gradient. Soil Biol Biochem 54:36–47
Ye R, Keller JK, Jin Q, Bohannan BJ, Bridgham SD (2016) Peatland types influence the inhibitory effects of a humic substance analog on methane production. Geoderma 265:131–140
Zhang L, Li J, Ban Q, He J, Jha AK (2012) Metabolic pathways of hydrogen production in fermentative acidogenic microflora. J Microbiol Biotechnol 22(5):668–673
Zhuang Q, Melillo JM, Sarofim MC, Kicklighter DW, McGuire AD, Felzer BS, Sokolov A, Prinn RG, Steudler PA, Hu S (2006) CO2 and CH4 exchanges between land ecosystems and the atmosphere in northern high latitudes over the 21st century. Geophys Res Lett 33(17):128
Zinder SH (1990) Conversion of acetic acid to methane by thermophiles. FEMS Microbiol Rev 6(2–3):125–137
Zinder SH (1994) Syntrophic acetate oxidation and “reversible acetogenesis”. In: Drake HL (eds) Acetogenesis. Chapman & hall microbiology series (Physiology / Ecology / Molecular Biology / Biotechnology). Springer, Boston, MA, pp 386–415
Zinder SH, Anguish T (1992) Carbon monoxide, hydrogen, and formate metabolism during methanogenesis from acetate by thermophilic cultures of Methanosarcina and Methanothrix strains. Appl Environ Microbiol 58(10):3323–3329
Acknowledgements
This study was funded by NSF ARC # 1304804 and Macrosystem Biology 1241937. This publication is dedicated to honor the late Mark E Hines, who influenced others with his unconditional love, unbelievable kindness and consideration. He will be forever missed as a friend, mentor, and incredible role model. We thank two anonymous reviewers for their thoughtful comments that greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, L., Liu, X., Duddleston, K. et al. The Effects of pH, Temperature, and Humic-Like Substances on Anaerobic Carbon Degradation and Methanogenesis in Ombrotrophic and Minerotrophic Alaskan Peatlands. Aquat Geochem 26, 221–244 (2020). https://doi.org/10.1007/s10498-020-09372-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-020-09372-0