Abstract
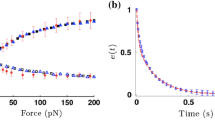
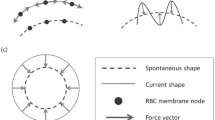
In this paper, we propose a multiscale numerical algorithm to simulate the hemolytic release of hemoglobin (Hb) from red blood cells (RBCs) flowing through sieves containing micropores with mean diameters smaller than RBCs. Analyzing the RBC damage in microfiltration is important in the sense that it can quantify the sensitivity of human erythrocytes to mechanical hemolysis while they undergo high shear rate and high deformation. Here, the numerical simulations are carried out via lattice Boltzmann method and spring connected network (SN) coupled by an immersed boundary method. To predict the RBC sublytic damage, a sub-cellular damage model derived from molecular dynamic simulations is incorporated in the cellular solver. In the proposed algorithm, the local RBC strain distribution calculated by the cellular solver is used to obtain the pore radius on the RBC membrane. Index of hemolysis (IH) is calculated by resorting to the resulting pore radius and solving a diffusion equation considering the effects of steric hinderance and increased hydrodynamic drag due to the size of the hemoglobin molecule. It should be mentioned that current computational hemolysis models usually utilize empirical fitting of the released free hemoglobin (Hb) in plasma from damaged RBCs. These empirical correlations contain ad hoc parameters that depend on specific device and operating conditions, thus cannot be used to predict hemolysis under different conditions. In contrast to the available hemolysis model, the proposed algorithm does not have any empirical parameters. Therefore, it can predict the IH in microfilter with different sieve pore sizes and filtration pressures. Also, in contrast to empirical correlations in which the Hb release is related to shear stress and exposure time without considering the physical processes, the proposed model links flow-induced deformation of the RBC membrane to membrane permeabilization and hemoglobin release. In this paper, the cellular solver is validated by simulating optical tweezers experiment, shear flow experiment as well as an experiment to measure RBC deformability in a very narrow microchannel. Moreover, the shape of a single RBC at the rupture moment is compared with corresponding experimental data. Finally, to validate the damage model, the results obtained from our parametric study on the role of filtration pressure and sieve pore size in Hb release are compared with experimental data. Numerical results are in good agreement with experimental data. Similar to the corresponding experiment, the numerical results confirm that hemolysis increases with increasing the filtration pressure and reduction in pore size on the sieve. While in experiment, the RBC pore size cannot be measured, the numerical results can quantify the RBC pore size. The numerical results show that at the sieve pore size of 2.2 µm above 25 cm Hg, RBC pore size is above 75 nm and RBCs experience rupture. More importantly, the results demonstrate that the proposed approach is independent from the operating conditions and it can estimate the hemolysis in a wide range of filtration pressure and sieve pore size with reasonable accuracy.















Similar content being viewed by others
References
Aaron N, Jacobson L (2015) Left ventricular assist device. In: Jacobson L, Okuda Y, Godwin S (eds) SimWars simulation case book: emergency medicine. Cambridge University Press, Cambridge, pp 104–107. https://doi.org/10.1017/CBO9781107111011.026
Abkarian M, Faivre M, Stone HA (2006) High-speed microfluidic differential manometer for cellular-scale hydrodynamics. Proc Natl Acad Sci 103(3):538–542
Abkarian M, Faivre M, Horton R, Smistrup K, Best-Popescu CA, Stone HA (2008) Cellular-scale hydrodynamics. Biomed Mater 3(3):34011–34013
Al Jarallah AS, Duncan WJ, Broecker L, Allen L, Cornel G (1997) The Hemopump as a left ventricular assist device in pediatric applications: initial Canadian applications. Can J Cardiol 13(5):489–494
Arora D (2005) Computational hemodynamics: hemolysis and viscoelasticity. Rice University, Houston
Arora D, Behr M, Pasquali M (2004) A tensor-based measure for estimating blood damage. Artif Organs 28(11):1002–1015
Arora D, Behr M, Pasquali M (2006) Hemolysis estimation in a centrifugal blood pump using a tensor-based measure. Artif Organs 30(7):539–547
Chen S, Doolen GD (1998) Lattice Boltzmann method for fluid flows. Annu Rev Fluid Mech 30(1):329–364
Chen Y, Sharp MK (2011) A strain-based flow-induced hemolysis prediction model calibrated by in vitro erythrocyte deformation measurements. Artif Organs 35(2):145–156
Chien S, Luse SA, Bryant CA (1971) Hemolysis during filtration through micropores: a scanning electron microscopic and hemorheologic correlation. J Microvasc Res 3:183–203
Davidson MG, Deen WM (1988) Hydrodynamic theory for the hindered transport of flexible macromolecules in porous membranes. J Memb Sci 35(2):167–192
Den Otter WK (2009) Free energies of stable and metastable pores in lipid membranes under tension. J Chem Phys 131(20):205101–205109
Doster W, Longeville S (2007) Microscopic diffusion and hydrodynamic interactions of hemoglobin in red blood cells. Biophys J 93(4):1360–1368
Ezzeldin HM, de Tullio MD, Vanella M, Solares SD, Balaras E (2015) A strain-based model for mechanical hemolysis based on a coarse-grained red blood cell model. Ann Biomed Eng 43(6):1398–1409
Faghih MM, Sharp MK (2019) Modeling and prediction of flow-induced hemolysis: a review. Biomech Model Mechanobiol 18(4):845–881
Farinas M-I, Garon A, Lacasse D, N’dri D (2006) Asymptotically consistent numerical approximation of hemolysis. J Biomech Eng 128(5):688
FDA (2013) Critical path. Computational fluid dynamics (CFD)/blood damage project. Fda
Giersiepen M, Wurzinger LJ, Opitz R, Reul H (1990) Estimation of shear stress-related blood damage in heart valve prostheses-in vitro comparison of 25 aortic valves. Int J Artif Organs 13(5):300–306
Grigioni M, Morbiducci U, D’Avenio G, Di Benedetto G, Del Gaudio C (2005) A novel formulation for blood trauma prediction by a modified power-law mathematical model. Biomech Model Mechanobiol 4(4):249–260
Heuser G, Opitz R (2017) A Couette viscometer for short time shearing of blood. Biorheology 17(1–2):17–24
Hung TC et al (1991) Effects of long-term Novacor artificial heart support on blood rheology. ASAIO Trans 37(3):M312–M313
Kormos RL, Borovetz HS, Griffith BP, Hung TC (1987) Rheologic abnormalities in patients with the Jarvik-7 total artificial heart. ASAIO Trans 33(3):413–417
Koshiyama K, Wada S (2011) Molecular dynamics simulations of pore formation dynamics during the rupture process of a phospholipid bilayer caused by high-speed equibiaxial stretching. J Biomech 44(11):2053–2058
Leontiadou H, Mark AE, Marrink SJ (2004) Molecular dynamics simulations of hydrophilic pores in lipid bilayers. Biophys J 86(4):2156–2164
Liu WK et al (2006) Immersed finite element method and its applications to biological systems. Comput Methods Appl Mech Eng 195(13–16):1722–1749
Long CC, Marsden AL, Bazilevs Y (2014) Shape optimization of pulsatile ventricular assist devices using FSI to minimize thrombotic risk. Comput Mech 54(4):921–932
Mills JP, Qie L, Dao M, Lim CT, Suresh S (2004) Nonlinear elastic and viscoelastic deformation of the human red blood cell with optical tweezers. Mech Chem Biosyst 1(3):169–180
Pauli L, Nam J, Pasquali M, Behr M (2013) Transient stress-based and strain-based hemolysis estimation in a simplified blood pump. Int j Numer Method Biomed Eng 29(10):1148–1160
Quinn DJ et al (2011) Combined simulation and experimental study of large deformation of red blood cells in microfluidic systems. Ann Biomed Eng 39(3):1041–1050
Rother RP, Bell L, Hillmen P, Gladwin MT (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. J Am Med Assoc 293(13):1653–1662
Sohrabi S, Liu Y (2017) A cellular model of shear-induced hemolysis. Artif Organs 41(9):80–91
Sohrabi S, Zheng J, Finol EA, Liu Y (2014) Numerical simulation of particle transport and deposition in the pulmonary vasculature. J Biomech Eng 136(12):121010
Succi S (2001) The lattice Boltzmann equation for fluid dynamics and beyond. Oxford University Press, Oxford
Tan J, Thomas A, Liu Y (2012) Influence of red blood cells on nanoparticle targeted delivery in microcirculation. Soft Matter 8(6):1934–1946
Tan J, Shah S, Thomas A, Ou-Yang HD, Liu Y (2013a) The influence of size, shape and vessel geometry on nanoparticle distribution. Microfluid Nanofluidics 232(3):502–514
Tan J, Wang S, Yang J, Liu Y (2013b) Coupled particulate and continuum model for nanoparticle targeted delivery. Comput Struct 122:128–134
Tan J, Keller W, Sohrabi S, Yang J, Liu Y (2016) Characterization of nanoparticle dispersion in red blood cell suspension by the lattice boltzmann-immersed boundary method. Nanomaterials 6(2):1–14
Tan J, Sohrabi S, He R, Liu Y (2018) Numerical simulation of cell squeezing through a micropore by the immersed boundary method. Proc Inst Mech Eng Part C J Mech Eng Sci 232(3):502–514
Taskin ME, Fraser KH, Zhang T, Wu C, Griffith BP, Wu ZJ (2012) Evaluation of Eulerian and Lagrangian models for hemolysis estimation. ASAIO J 58(4):363–372
Tolpekina TV, Den Otter WK, Briels WJ (2004) Simulations of stable pores in membranes: System size dependence and line tension. J Chem Phys 121(10):154701–154905
Tomasini MD, Rinaldi C, Tomassone MS (2010) Molecular dynamics simulations of rupture in lipid bilayers. Exp Biol Med 235(2):181–188
Vitale F et al (2014) A multiscale, biophysical model of flow-induced red blood cell damage. AIChE J 60(4):1509–1516
Wood HG, Throckmorton AL, Untaroiu A, Song X (2005) The medical physics of ventricular assist devices. Rep Prog Phys 68(3):545–576
Wu J, Yun BM, Fallon AM, Hanson SR, Aidun CK, Yoganathan AP (2011) Numerical investigation of the effects of channel geometry on Platelet activation and blood damage. Ann Biomed Eng 39(2):897–910
Yao W et al (2001) Low viscosity Ektacytometry and its validation tested by flow chamber. J Biomech 34(11):1501–1509
Yeleswarapu KK, Antaki JF, Kameneva MV, Rajagopal KR (1995) A mathematical model for shear-induced hemolysis. Artif Organs 19(7):576–582
Yu H, Engel S, Janiga G, Thévenin D (2017) A review of hemolysis prediction models for computational fluid dynamics. Artif Organs 41(7):603–621
Závodszky G, van Rooij B, Azizi V, Hoekstra A (2017) Cellular level in-silico modeling of blood rheology with an improved material model for red blood cells. Front Physiol 8:563
Zhang T et al (2012) Study of flow-induced hemolysis using novel couette-type blood-shearing devices. Artif Organs 35(12):1180–1186
Zou Q, He X (1997) On pressure and velocity boundary conditions for the lattice Boltzmann BGK model. Phys Fluids 9(6):1591–1598
Acknowledgements
This work was supported by National Institutes of Health (NIH) Grant number R01HL131750.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikfar, M., Razizadeh, M., Paul, R. et al. Multiscale modeling of hemolysis during microfiltration. Microfluid Nanofluid 24, 33 (2020). https://doi.org/10.1007/s10404-020-02337-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-020-02337-3