Abstract
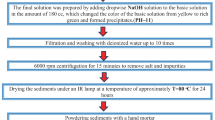
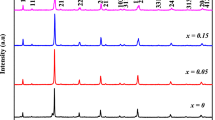
Gadolinium doped tin oxide–iron oxide nanoparticles (Gd/Fe1.727Sn0.205O3) were synthesized via sol–gel method followed by hydrothermal method. Ethylene glycol played the role of directing agent to control surface morphology. Physical and optical properties of Gd/Fe1.727Sn0.205O3 nanoparticles were studied as a function of calcination temperature. Characterization techniques like thermogravimetric analysis (TGA), fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), particle size analyzer (PSA), scanning electron microscopy (SEM), transmission electron microscopy (TEM), UV–Visible spectroscopy (UV–VIS), and four-point probe technique have been used to study the thermal degradation, kinetics, thermodynamic properties, structural analysis, surface morphology, optical and electrical properties of nanoparticles. Prominent peaks in FTIR spectra at 563, 418, and 542 cm−1 were observed for Fe–O, Sn–O, and Gd–O, respectively. The uncalcined nanoparticles follow first order kinetics and Freeman–Carrol method was applied for calculating the activation energy. It was observed that nanoparticles calcined at 700 °C have 8.9 nm particle size calculated with particle size analyzer, which is smallest among all and having band gap energy of 2.3 eV. SEM micrographs show hexagonal geometry. The dependence of electrical resistance on temperature shows that these nanoparticles possess semiconducting behavior. These nanoparticles can be used as cathode material for solid oxides fuel cells (SOFCs) application. The nanoparticles calcined at 700 °C showed highest power density of 83.27 W cm−2 at 650 °C with open current voltage of 0.793 V.

Highlights
-
Novel high surface area of Gd/Fe1.727Sn0.205O3 nanoparticles were synthesized using sol–gel and hydrothermal methods.
-
The smallest band gap and particle size was observed for the sample calcined at 700 °C.
-
Four probe dc conductivity value recoded was 0.709 mS/cm for sample calcined at 700 °C.
-
Solid oxides fuel cell (SOFC) demonstrates that Gd/Fe1.727Sn0.205O3 as cathode performed better when calcined at 700 °C with high power density of 83.27 W cm−2.














Similar content being viewed by others
References
Carrette L, Friedrich KA, Stimming U (2001) Furl cells—fundamentals and application. Fuel Cells 1:5–39
Carrette L, Friedrich KA, Stimming U (2000) Fuel cells: principles, types, fuels, and applications. ChemPhysChem 1:162–193
Raza R, Wang X, Ma Y, Liu X, Zhu B (2010) Improved ceria–carbonate composite electrolytes. Int J Hydrog Energy 35:2684–2688
Liangdong F, Bin Z, Mingming C, Chengyang W, Rizwan R, Haiying Q, Xuetao W, Xiaodi W, Ying M (2012) High performance transition metal oxide composite cathode for low temperature solid oxide fuel cells. J Power Sources 203:65–71
Rizwan R, Nadeem A, Muhammad Sufyan J, Asia R, Ullah K, Ali A, Saleem M, Ahmed R (2016) Fuel cell technology for sustainable development in Pakistan—an over-view. Renew Sust Energy Rev 53:450–461
Javaid S, Farrukh MA, Muneer I, Shahid M, Khaleeq-ur-Rahman M, Umar AA (2015) Influence of optical band gap and particle size on the catalytic properties of Sm/SnO2–TiO2 nanoparticles. Superlattices Microstruct 82:234–247
Muneer I, Farrukh MA, Javaid S, Shahid M, Khaleeq-ur-Rahman M (2015) Synthesis of Gd2O3/Sm2O3 nanocomposite via sonication and hydrothermal methods and its optical properties. Superlattices Microstruct 77:256–266
Yazid H, Adnan R, Farrukh MA, Hamid SA (2011) Synthesis of u/Al2O3 nanocatalyst and its application in the reduction of p-nitrophenol. J Chin Chem Soc 58:593–601
Farrukh MA, Muneer I, Butt KM, Batool S, Fakhar N (2016) Effect of dielectric constant of solvents on the particle size and bandgap of La/SnO2-TiO2 nanoparticles and their catalytic properties. J Chin Chem Soc 63:952–959
Häber T, Schmitt U, Emmeluth C, Suhm MA (2001) Ragout-jet FTIR spectroscopy of cluster isomerism and cluster dynamics: from carboxylic acid dimers to N2O nanoparticles. Faraday Discuss 118:331–359
Farrukh MA, Butt KM, Chong K, Chang WS (2019) Photoluminescence emission behavior on the reduced band gap of Fe doping in CeO2–SiO2 nanocomposite and photophysical properties. J Saudi Chem Soc 23:561–575
Arshad M, Abbas M, Ehtisham-ul-Haque S, Farrukh MA, Ali A, Rizvi H, Soomro G, Afshan G, Yameen MA, Iqbal M (2019) Synthesis and characterization of SiO2 doped Fe2O3 nanoparticles: photocatalytic and antimicrobial activity evaluation. J Mol Struct 1180:244–250
Afzaal A, Farrukh MA (2017) Zwitterionic surfactant assisted synthesis of Fe doped SnO2-SiO2 nanocomposite with enhanced photocatalytic activity under sun light. Mater Sci Eng B 223:167–177
Shahid M, Farrukh MA, Umar AA, Khaleeq-ur-Rahman M (2014) Solvent controlled synthesis of CaO–MgO nanocomposites and their application in the photodegradation of organic pollutants of industrial waste. Russ J Phys Chem A 88:836–844
Sahai A, Goswami N (2014) Structural and vibrational properties of ZnO nanoparticles synthesized by the chemical precipitation method. Phys E: Low-Dimens Syst Nanostruct 58:130–137
Perveen S, Farrukh MA (2017) Influence of lanthanum precursors on the heterogeneous La/SnO2–TiO2 nanocatalyst with enhanced catalytic activity under visible light. J Mater Sci: Mater Electron 28:10806–10818
Arjmandi R, Hassan A, Haafiz MKM, Zakaria Z, Islam MS (2016) Effect of hydrolysed cellulose nanowhiskers on properties of montmorillonite/polylactic acid nanocomposites. Int J Biol Macromol 82:998–1010
Çepelioğullar O, Haykırı-Açma H, Yaman S (2016) Kinetic modelling of RDF pyrolysis: model-fitting and model-free approaches. Waste Manag 48:275–284
Zhang J, Chen DH, Yuan Y, Gong Y (2001) Thermal decomposition kinetics of ribavirin and its stability. Yao Xue Xue Bao 36:452–455
Wei B, Li H, Tian Y, Xu X, Jin Z (2015) Thermal degradation behavior of hypochlorite-oxidized starch nanocrystals under different oxidized levels. Carbohydr Polym 124:124–130
Del MG, María R, Amalia M, Muñoz M, María L (2005) Micellar solutions of sulfobetaine surfactants in water−ethylene glycol mixtures: surface tension, fluorescence, spectroscopic, conductometric, and kinetic studies. Langmuir 21:7161–7169
Ali S, Farrukh MA (2018) Effect of calcination temperature on the structural, thermodynamic, and optical properties of MoO3 nanoparticles. J Chin Chem Soc 65:276–288
Ali D, Butt MZ (2014) Structural characteristics and inverse Hall–Petch relation in high-purity nickel irradiated with nanosecond infrared laser pulses. Physica B 444:77–84
Ali D, Butt MZ, Muneer I, Bashir F, Saleem M (2017) Correlation between structural and optoelectronic properties of tin doped indium oxide thin films. Optik 128:235–246
Lim J, Yeap SP, Che HX, Low SC (2013) Characterization of magnetic nanoparticle by dynamic light scattering. Nano Lett 8:381
Loyalka SK, Riggs CA (1995) Inverse problem in diffuse reflectance spectroscopy: accuracy of the Kubelka-Munk equations. Appl Spectrosc 49:1107–1110
Pecho OE, Ghinea R, Ionescu AM, Cardona JC, Della B, Pérez MD (2015) Optical behavior of dental zirconia and dentin analyzed by Kubelka–Munk theory. Dent Mater 31:60–67
Fujisawa J-i, Eda T, Hanaya M (2017) Comparative study of conduction-band and valence-band edges of TiO2, SrTiO3, and BaTiO3 by ionization potential measurements. Chem Phys Lett 685:23–26
El-Sheikh SM, Khedr TM, Zhang G, Vogiazi V, Ismail AA, O’Shea K, Dionysiou D (2017) Tailored synthesis of anatase–brookite heterojunction photocatalysts for degradation of cylindrospermopsin under UV–Vis light. Chem Eng J 310:428–436
Al-Douri Y, Badi N, Voon CH (2018) Synthesis of carbon-based quantum dots from starch extracts: optical investigations. Luminescence 33:260–266
Tripathy SK, Pattanaik A (2016) Optical and electronic properties of some semiconductors from energy gaps. Opt Mater 53:123–133
Mushtaq N, Xia C, Dong W, Abbas G, Raza R, Ali A, Rauf S, Wang B, Kim J-S, Zhu B (2018) Perovskite SrFe1-xTixO3-δ (x<= 0.1) cathode for low temperature solid oxide fuel cell. Ceram Int 44:10266–10272
Mi Y, Xia C, Zhu B, Raza R, Afzal M, Riess I (2018) Experimental and physical approaches on a novel semiconducting-ionic membrane fuel cell. Int J Hydrog Energy 43:12756–12764
Arshad MS, Raza R, Ahmad MA, Abbas G, Ali A, Rafique A, Ullah MK, Rauf S, Asghar MI, Mushtaq N, Shahid SA, Naseem S (2018) An efficient Sm and Ge co-doped ceria nanocomposite electrolyte for low temperature solid oxide fuel cells. Ceram Int 44:170–174
Li W, Liang C, Zhou W, Qiu J, Zhou SG, Xin Q (2003) Preparation and characterization of multiwalled carbon nanotube-supported platinum for cathode catalysts of direct methanol fuel cells. J Phy Chem B 107:6292–6299
Choi HJ, Kim M, Neoh KC, Jang DY, Kim HJ, Shin JM, Kim G-T, Shim JH (2017) High-performance silver cathode surface treated with scandia-stabilized zirconia nanoparticles for intermediate temperature solid oxide fuel cells. Adv Energy Mater 7:1601956
Acknowledgements
The corresponding author (Muhammad Akhyar Farrukh) would like to thank Higher Education Commission (HEC) Pakistan for providing funds through Project Nos. 20-3142/NRPU/R&D/HEC/ and 20-2660/NRPU/R&D/HEC/ and The World Academy of Sciences (TWAS), Italy. TWAS Research Grant No. 11-028 RG/MSN/AS_C to establish Nano-Chemistry Lab.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Muneer, I., Farrukh, M.A. & Raza, R. Influence of annealing temperature on the physical and photoelectric properties of Gd/Fe1.727Sn0.205O3 nanoparticles for solid oxides fuel cell application. J Sol-Gel Sci Technol 94, 98–108 (2020). https://doi.org/10.1007/s10971-019-05168-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05168-z