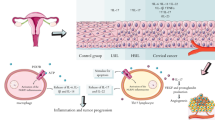
Abstract
Cervical cancer is the fourth most common type of cancer incidence in the world female population, and it has become a public health problem worldwide. Several factors are involved in this type of cancer, including intrinsic factors related to the inflammatory process, such as extracellular nucleotides and adenosine—components of the purinergic system. The present review focuses on the role of the purinergic system in cervical cancer, especially regarding the interaction of extracellular nucleotides with their respective receptors expressed in the tumor microenvironment of cervical cancer and their role in the host immune response. The high concentrations of extracellular nucleotides in the tumor microenvironment of cervical cancer interfere in the regulation, proliferation, differentiation, and apoptosis of cancer cells of the uterine cervix through different P1 and P2 receptor subtypes. Such diverse cellular processes that are mediated by adenosine triphosphate and adenosine across the tumor microenvironment and that also have effects on host immune defense will be reviewed here in detail.



Similar content being viewed by others
References
Cohen PA, Jhingran A, Oaknin A, Denny L (2019) Cervical cancer. Lancet 393:169–182. https://doi.org/10.1016/S0140-6736(18)32470-X
Campion M, Canfell K (2015) Cervical Cancer screening and Preinvasive disease. In: Gynecologic oncology, 6th edn. Wolters Kluwer, Filadélfia, pp 242–325
Chen L, Luan S, Xia B, Liu Y, Gao Y, Yu H, Mu Q, Zhang P, Zhang W, Zhang S, Wei G, Yang M, Li K (2018) Integrated analysis of HPV-mediated immune alterations in cervical cancer. Gynecol Oncol 149:248–255. https://doi.org/10.1016/j.ygyno.2018.01.031
Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE (2011) Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst 103:368–383. https://doi.org/10.1093/jnci/djq562
Schiffman M, Doorbar J, Wentzensen N et al (2016) Carcinogenic human papillomavirus infection. Nat Rev Dis Primers 2:16086. https://doi.org/10.1038/nrdp.2016.86
Chan CK, Aimagambetova G, Ukybassova T, Kongrtay K, Azizan A (2019) Human papillomavirus infection and cervical cancer: epidemiology, screening, and vaccination—review of current perspectives. J Oncol 2019:3257939. https://doi.org/10.1155/2019/3257939
Di Virgilio F, Sarti AC, Falzoni S et al (2018) Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer 18:601–618. https://doi.org/10.1038/s41568-018-0037-0
Gao Z, Dong K, Zhang H (2014) The roles of CD73 in Cancer. Biomed Res Int 2014:460654. https://doi.org/10.1155/2014/460654
Ferrari D, Malavasi F, Antonioli L (2017) A purinergic trail for metastases. Trends Pharmacol Sci 38:277–290. https://doi.org/10.1016/j.tips.2016.11.010
Di Virgilio F (2012) Purines, purinergic receptors, and cancer. Cancer Res 72:5441–5447. https://doi.org/10.1158/0008-5472.CAN-12-1600
Li X, Gong Z, Zhang L, Zhao C, Zhao X, Gu X, Chen H (2015) Autophagy knocked down by high-risk HPV infection and uterine cervical carcinogenesis. Int J Clin Exp Med 8:10304–10314
de Andrade Mello P, Filippi-Chiela EC, Nascimento J et al (2014) Adenosine uptake is the major effector of extracellular ATP toxicity in human cervical cancer cells. Mol Biol Cell 25:2905–2918. https://doi.org/10.1091/mbc.e14-01-0042
Lee SG, Choi J-K, Choi BH et al (2006) The effect of adenosine 5′-triphosphate on calcium mobilization and cell proliferation in cervical cancer cells. Eur J Obstet Gynecol Reprod Biol 127:110–114. https://doi.org/10.1016/j.ejogrb.2004.07.030
Adinolfi E, Raffaghello L, Giuliani AL, Cavazzini L, Capece M, Chiozzi P, Bianchi G, Kroemer G, Pistoia V, di Virgilio F (2012) Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res 72:2957–2969. https://doi.org/10.1158/0008-5472.CAN-11-1947
Di Virgilio F, Adinolfi E (2017) Extracellular purines, purinergic receptors and tumor growth. Oncogene 36:293–303. https://doi.org/10.1038/onc.2016.206
Burnstock G, Knight GE (2018) The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal 14:1–18. https://doi.org/10.1007/s11302-017-9593-0
Zhang L, Jiang Y, Lu X, Zhao H, Chen C, Wang Y, Hu W, Zhu Y, Yan H, Yan F (2018) Genomic characterization of cervical cancer based on human papillomavirus status. Gynecol Oncol 152:629–637. https://doi.org/10.1016/j.ygyno.2018.12.017
Fernandes JV, de Medeiros Fernandes TAA, de Azevedo JCV et al (2015) Link between chronic inflammation and human papillomavirus-induced carcinogenesis. Oncol Lett 9:1015–1026. https://doi.org/10.3892/ol.2015.2884
Alizon S, Murall CL, Bravo IG (2017) Why human papillomavirus acute infections matter. Viruses 9:293. https://doi.org/10.3390/v9100293
Boda D, Docea A, Calina D, Ilie MA, Caruntu C, Zurac S, Neagu M, Constantin C, Branisteanu DE, Voiculescu V, Mamoulakis C, Tzanakakis G, Spandidos DA, Drakoulis N, Tsatsakis AM (2018) Human papilloma virus: apprehending the link with carcinogenesis and unveiling new research avenues (review). Int J Oncol 52:637–655. https://doi.org/10.3892/ijo.2018.4256
Okunade KS (2019) Human papillomavirus and cervical cancer. J Obstet Gynaecol:1–7. https://doi.org/10.1080/01443615.2019.1634030
Georgescu SR, Mitran CI, Mitran MI et al (2018) New insights in the pathogenesis of HPV infection and the associated carcinogenic processes: the role of chronic inflammation and oxidative stress. J Immunol Res 2018:1–10. https://doi.org/10.1155/2018/5315816
Krump NA, You J (2018) Molecular mechanisms of viral oncogenesis in humans. Nat Rev Microbiol 16:684–698. https://doi.org/10.1038/s41579-018-0064-6
Banerjee NS, Wang H-K, Broker TR, Chow LT (2011) Human papillomavirus (HPV) E7 induces prolonged G2 following S phase reentry in differentiated human keratinocytes. J Biol Chem 286:15473–15482. https://doi.org/10.1074/jbc.M110.197574
Williams VM, Filippova M, Soto U, Duerksen-Hughes PJ (2011) HPV-DNA integration and carcinogenesis: putative roles for inflammation and oxidative stress. Futur Virol 6:45–57. https://doi.org/10.2217/fvl.10.73
Herbster S, Paladino A, Freitas S, Boccardo E (2018) Alterations in the expression and activity of extracellular matrix components in HPV-associated infections and diseases. Clinics 73:–e551s. https://doi.org/10.6061/clinics/2018/e551s
Wang Q, Wang L, Feng Y-H et al (2004) P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am J Physiol-Cell Physiol 287:1349–1358. https://doi.org/10.1152/ajpcell.00256.2004
Maldonado PA, Pimentel VC, Negrini LA, Morsch VM, Schetinger MR (2012) Role of the purinergic system in patients with cervical intraepithelial neoplasia and uterine cancer. Biomed Pharmacother 66:6–11. https://doi.org/10.1016/j.biopha.2011.09.007
Bahreyni A, Samani SS, Ghorbani E, Rahmani F, Khayami R, Toroghian Y, Behnam-Rassouli R, Khazaei M, Ryzhikov M, Parizadeh MR, Hasanzadeh M, Avan A, Hassanian SM (2018) Adenosine: an endogenous mediator in the pathogenesis of gynecological cancer. J Cell Physiol 233:2715–2722. https://doi.org/10.1002/jcp.26056
Burnstock G (2014) Purinergic signalling in the reproductive system in health and disease. Purinergic Signal 10:157–187. https://doi.org/10.1007/s11302-013-9399-7
Alcocer-González JM, Berumen J, Taméz-Guerra R, Bermúdez-Morales V, Peralta-Zaragoza O, Hernández-Pando R, Moreno J, Gariglio P, Madrid-Marina V (2006) In vivo expression of immunosuppressive cytokines in human papillomavirus-transformed cervical cancer cells. Viral Immunol 19:481–491. https://doi.org/10.1089/vim.2006.19.481
Torres-Poveda K, Bahena-Román M, Madrid-González C, Burguete-García AI, Bermúdez-Morales VH, Peralta-Zaragoza O, Madrid-Marina V (2014) Role of IL-10 and TGF-β1 in local immunosuppression in HPV-associated cervical neoplasia. World J Clin Oncol 5:753–763. https://doi.org/10.5306/wjco.v5.i4.753
Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren GJ (2008) Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother 57:197–206. https://doi.org/10.1007/s00262-007-0362-8
Mora-García ML, Ávila-Ibarra LR, García-Rocha R, Weiss-Steider B, Hernández-Montes J, Don-López CA, Gutiérrez-Serrano V, Titla-Vilchis IJ, Fuentes-Castañeda MC, Monroy-Mora A, Jave-Suárez LF, Chacón-Salinas R, Vallejo-Castillo L, Pérez-Tapia SM, Monroy-García A (2017) Cervical cancer cells suppress effector functions of cytotoxic T cells through the adenosinergic pathway. Cell Immunol 320:46–55. https://doi.org/10.1016/j.cellimm.2017.09.002
Gutiérrez-Hoya A, Zerecero-Carreón O, Valle-Mendiola A, Moreno-Lafont M, López-Santiago R, Weiss-Steider B, Soto-Cruz I (2019) Cervical Cancer cells express markers associated with immunosurveillance. J Immunol Res 2019:1242979. https://doi.org/10.1155/2019/1242979
Patel S, Chiplunkar S (2009) Host immune responses to cervical cancer. Curr Opin Obstet Gynecol 21:54–59. https://doi.org/10.1097/GCO.0b013e32831a9890
Wang J, Matosevic S (2018) Adenosinergic signaling as a target for natural killer cell immunotherapy. J Mol Med (Berl) 96:903–913. https://doi.org/10.1007/s00109-018-1679-9
Regateiro FS, Cobbold SP, Waldmann H (2013) CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol 171:1–7. https://doi.org/10.1111/j.1365-2249.2012.04623.x
García-Rocha R, Monroy-García A, Hernández-Montes J, Weiss-Steider B, Gutiérrez-Serrano V, del Carmen Fuentes-Castañeda M, Ávila-Ibarra LR, Don-López CA, Torres-Pineda DB, de Lourdes Mora-García M (2019) Cervical cancer cells produce TGF-β1 through the CD73-adenosine pathway and maintain CD73 expression through the autocrine activity of TGF-β1. Cytokine 118:71–79. https://doi.org/10.1016/j.cyto.2018.09.018
Burnstock G (1997) The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology 36:1127–1139. https://doi.org/10.1016/s0028-3908(97)00125-1
White N, Burnstock G (2006) P2 receptors and cancer. Trends Pharmacol Sci 27:211–217. https://doi.org/10.1016/j.tips.2006.02.004
Bagatini MD, dos Santos AA, Cardoso AM et al (2018) The impact of purinergic system enzymes on noncommunicable, neurological, and degenerative diseases. J Immunol Res 2018:4892473. https://doi.org/10.1155/2018/4892473
Burnstock G, Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304. https://doi.org/10.1016/S0074-7696(04)40002-3
Burnstock G (2017) Purinergic signalling: therapeutic developments. Front Pharmacol 8:661. https://doi.org/10.3389/fphar.2017.00661
Graner MW (2018) Extracellular vesicles in cancer immune responses: roles of purinergic receptors. Semin Immunopathol 40:465–475. https://doi.org/10.1007/s00281-018-0706-9
Gessi S, Merighi S, Sacchetto V et al (2011) Adenosine receptors and cancer. Biochim Biophys Acta Biomembr 1808:1400–1412. https://doi.org/10.1016/j.bbamem.2010.09.020
Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K (2018) Pharmacology of adenosine receptors: the state of the art. Physiol Rev 98:1591–1625. https://doi.org/10.1152/physrev.00049.2017
Burnstock G, Di Virgilio F (2013) Purinergic signalling and cancer. Purinergic Signal 9:491–540. https://doi.org/10.1007/s11302-013-9372-5
von Kügelgen I, Wetter A (2000) Molecular pharmacology of P2Y-receptors. Naunyn Schmiedeberg's Arch Pharmacol 362:310–323. https://doi.org/10.1007/s002100000310
Jacobson KA, Paoletta S, Katritch V, Wu B, Gao ZG, Zhao Q, Stevens RC, Kiselev E (2015) Nucleotides acting at P2Y receptors: connecting structure and function. Mol Pharmacol 88:220–230. https://doi.org/10.1124/mol.114.095711
Robson SC, Sévigny J, Zimmermann H (2006) The E-NTPDase family of ectonucleotidases: structure function relationships and pathophysiological significance. Purinergic Signal 2:409–430. https://doi.org/10.1007/s11302-006-9003-5
Yegutkin GG (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta 1783:673–694. https://doi.org/10.1016/j.bbamcr.2008.01.024
Vigano S, Alatzoglou D, Irving M, Ménétrier-Caux C, Caux C, Romero P, Coukos G (2019) Targeting adenosine in cancer immunotherapy to enhance T-cell function. Front Immunol 10:925. https://doi.org/10.3389/fimmu.2019.00925
Vijayan D, Young A, Teng MWL, Smyth MJ (2017) Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer 17:709–724. https://doi.org/10.1038/nrc.2017.86
de Andrade MP, Coutinho-Silva R, Savio LEB (2017) Multifaceted effects of extracellular adenosine triphosphate and adenosine in the tumor–host interaction and therapeutic perspectives. Front Immunol 8:1526. https://doi.org/10.3389/fimmu.2017.01526
Leone RD, Emens LA (2018) Targeting adenosine for cancer immunotherapy. J Immunother Cancer 6:57. https://doi.org/10.1186/s40425-018-0360-8
Zimmermann H, Zebisch M, Sträter N (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502. https://doi.org/10.1007/s11302-012-9309-4
Antonioli L, Pacher P, Vizi ES, Haskó G (2013) CD39 and CD73 in immunity and inflammation. Trends Mol Med 19:355–367. https://doi.org/10.1016/j.molmed.2013.03.005
Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A (2013) ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene 32:1743–1751. https://doi.org/10.1038/onc.2012.269
Huang Y, Gu Z, Fan Y, Zhai G, Zhao X, Sun Q, Shi Y, Lin G (2019) Inhibition of the adenosinergic pathway: the indispensable part of oncological therapy in the future. Purinergic Signal 15:53–67. https://doi.org/10.1007/s11302-018-9641-4
Gorodeski GI, Burfeind P, Gan SU et al (1998) Regulation by retinoids of P2Y2 nucleotide receptor mRNA in human uterine cervical cells. Am J Physiol 275:C758–C765. https://doi.org/10.1152/ajpcell.1998.275.3.C758
Welter-Stahl L, da Silva CM, Schachter J et al (2009) Expression of purinergic receptors and modulation of P2X7 function by the inflammatory cytokine IFNγ in human epithelial cells. Biochim Biophys Acta Biomembr 1788:1176–1187. https://doi.org/10.1016/j.bbamem.2009.03.006
Gorodeski GI (2002) Expression, regulation, and function of P2X4 purinergic receptor in human cervical epithelial cells. Am J Physiol-Cell Physiol 282:C84–C93. https://doi.org/10.1152/ajpcell.2002.282.1.C84
Gorodeski GI, Hopfer U, De Santis BJ et al (1995) Biphasic regulation of paracellular permeability in human cervical cells by two distinct nucleotide receptors. Am J Physiol-Cell Physiol 268:C1215–C1226. https://doi.org/10.1152/ajpcell.1995.268.5.C1215
Gorodeski GI, Goldfarb J (1997) Extracellular ATP regulates transcervical permeability by modulating two distinct paracellular pathways. Am J Physiol-Cell Physiol 272:C1602–C1610. https://doi.org/10.1152/ajpcell.1997.272.5.C1602
Gorodeski GI (2008) Regulation of paracellular permeability in low-resistance human vaginal-cervical epithelia. In: Ehrhardt C, Kim K-J (eds) Drug Absorption Studies: In Situ, In Vitro and In Silico Models, 1st edn. Springer Science & Business Media, pp 339–367
Darville T, Welter-Stahl L, Cruz C et al (2007) Effect of the purinergic receptor P2X7 on chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol 179:3707–3714. https://doi.org/10.4049/jimmunol.179.6.3707
Roger S, Jelassi B, Couillin I et al (2015) Understanding the roles of the P2X7 receptor in solid tumour progression and therapeutic perspectives. Biochim Biophys Acta Biomembr 1848:2584–2602. https://doi.org/10.1016/j.bbamem.2014.10.029
Zhang Y, Ding J, Wang L (2019) The role of P2X7 receptor in prognosis and metastasis of colorectal cancer. Adv Med Sci 64:388–394. https://doi.org/10.1016/j.advms.2019.05.002
Li X, Qi X, Zhou L, Catera D, Rote NS, Potashkin J, Abdul-Karim FW, Gorodeski GI (2007) Decreased expression of P2X7 in endometrial epithelial pre-cancerous and cancer cells. Gynecol Oncol 106:233–243. https://doi.org/10.1016/j.ygyno.2007.03.032
Feng Y-H, Li X, Wang L, Zhou L, Gorodeski GI (2006) A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem 281:17228–17237. https://doi.org/10.1074/jbc.M602999200
Li X, Zhou L, Feng Y-H, Abdul-Karim FW (2006) The P2X7 receptor: a novel biomarker of uterine epithelial cancers. Cancer Epidemiol Biomark Prev 15:1906–19013
Mackenzie AB, Young MT, Adinolfi E, Surprenant A (2005) Pseudoapoptosis induced by brief activation of ATP-gated P2X7 receptors. J Biol Chem 280:33968–33976. https://doi.org/10.1074/jbc.M502705200
Savio LEB, de Andrade MP, da Silva CG, Coutinho-Silva R (2018) The P2X7 receptor in inflammatory diseases: angel or demon? Front Pharmacol 9:52. https://doi.org/10.3389/fphar.2018.00052
Yang Y-C, Chang T-Y, Chen T-C, Lin WS, Chang SC, Lee YJ (2016) Functional variant of the P2X7 receptor gene is associated with human papillomavirus-16 positive cervical squamous cell carcinoma. Oncotarget 7:82798–82803. https://doi.org/10.18632/oncotarget.12636
Sluyter R (2017) The P2X7 receptor. Adv Exp Med Biol 1051:17–53. https://doi.org/10.1007/5584_2017_59
Di Virgilio F, Giuliani AL, Vultaggio-Poma V et al (2018) Non-nucleotide agonists triggering P2X7 receptor activation and pore formation. Front Pharmacol 9:39. https://doi.org/10.3389/fphar.2018.00039
Pevarello P, Bovolenta S, Tarroni P, Za L, Severi E, Torino D, Vitalone R (2017) P2X7 antagonists for CNS indications: recent patent disclosures. Pharm Pat Anal 6:61–76. https://doi.org/10.4155/ppa-2016-0044
Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, Pinton P, Rizzuto R, di Virgilio F (2005) Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell 16:3260–3272. https://doi.org/10.1091/mbc.e04-11-1025
De Marchi E, Orioli E, Dal Ben D, Adinolfi E (2016) P2X7 receptor as a therapeutic target. In: Advances in protein chemistry and structural biology, 1st edn. Elsevier, Epub, pp 39–79
Mendez LE, Manci N, Cantuaria G, Gomez-Marin O, Penalver M, Braunschweiger P, Nadji M (2002) Expression of glucose transporter-1 in cervical cancer and its precursors. Gynecol Oncol 86:138–143. https://doi.org/10.1006/gyno.2002.6745
Gorodeski GI (2012) P2X7 receptors and epithelial cancers. Wiley Interdiscip Rev Membr Transp Signal 1:349–371. https://doi.org/10.1002/wmts.33
Feng YH, Li X, Zeng R, Gorodeski GI (2006) Endogenously expressed truncated P2X7 receptor lacking the C-terminus is preferentially upregulated in epithelial cancer cells and fails to mediate ligand-induced pore formation and apoptosis. Nucleosides Nucleotides Nucleic Acids 25:1271–1276. https://doi.org/10.1080/15257770600890921
Wiley JS, Sluyter R, Gu BJ, Stokes L, Fuller SJ (2011) The human P2X7 receptor and its role in innate immunity. Tissue Antigens 78:321–332. https://doi.org/10.1111/j.1399-0039.2011.01780.x
Gorodeski GI (2004) Estrogen attenuates P2X7-R-mediated apoptosis of uterine cervical cells by blocking calcium influx. Nucleosides Nucleotides Nucleic Acids 23:1287–1293. https://doi.org/10.1081/NCN-200027549
Bukhari M, Deng H, Jones N, Towne Z, Woodworth CD, Samways DS (2015) Selective permeabilization of cervical cancer cells to an ionic DNA-binding cytotoxin by activation of P2Y receptors. FEBS Lett 589:1498–1504. https://doi.org/10.1016/j.febslet.2015.04.044
Muscella A, Elia MG, Greco S, Storelli C, Marsigliante S (2003) Activation of P2Y2 purinoceptor inhibits the activity of the Na+/K+-ATPase in HeLa cells. Cell Signal 15:115–121. https://doi.org/10.1016/S0898-6568(02)00062-1
Erb L, Weisman GA (2012) Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip Rev Membr Transp Signal 1:789–803. https://doi.org/10.1002/wmts.62
Okuda A, Furuya K, Kiyohara T (2003) ATP-induced calcium oscillations and change of P2Y subtypes with culture conditions in HeLa cells. Cell Biochem Funct 21:61–68. https://doi.org/10.1002/cbf.992
Locovei S, Wang J, Dahl G (2006) Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580:239–244. https://doi.org/10.1016/j.febslet.2005.12.004
Landecker H (2000) Immortality, in vitro: a history of the HeLa cell line creator. In: Biotechnology and culture: bodies, anxieties, ethics, 1st edn. Indiana University Press, Indiana, pp 53–72
Muscella A, Elia MG, Greco S, Storelli C, Marsigliante S (2003) Activation of P2Y2 receptor induces c-FOS protein through a pathway involving mitogen-activated protein kinases and phosphoinositide 3-kinases in HeLa cells. J Cell Physiol 195:234–240. https://doi.org/10.1002/jcp.10242
Durlacher CT, Chow K, Chen X-W, He ZX, Zhang X, Yang T, Zhou SF (2015) Targeting Na+/K+-ATPase in cancer treatment. Clin Exp Pharmacol Physiol 42:427–443. https://doi.org/10.1111/1440-1681.12385
Blok LJ, Chang GTG, Steenbeek-Slotboom M, van Weerden W, Swarts HG, de Pont JJ, van Steenbrugge G, Brinkmann AO (1999) Regulation of expression of Na+,K+-ATPase in androgen-dependent and androgen-independent prostate cancer. Br J Cancer 81:28–36. https://doi.org/10.1038/sj.bjc.6690647
Buvinic S, Bravo-Zehnder M, Boyer JL, Huidobro-Toro JP, González A (2007) Nucleotide P2Y1 receptor regulates EGF receptor mitogenic signaling and expression in epithelial cells. J Cell Sci 120:4289–4301. https://doi.org/10.1242/jcs.03490
Wee P, Wang Z (2017) Epidermal growth factor receptor cell proliferation signaling pathways. Cancers 9:52. https://doi.org/10.3390/cancers9050052
Gendaszewska-Darmach E, Szustak M (2016) Thymidine 5’-O-monophosphorothioate induces HeLa cell migration by activation of the P2Y6 receptor. Purinergic Signal 12:199–209. https://doi.org/10.1007/s11302-015-9492-1
Muscella A, Greco S, Elia MG, Storelli C, Marsigliante S (2004) Differential signalling of purinoceptors in HeLa cells through the extracellular signal-regulated kinase and protein kinase C pathways. J Cell Physiol 200:428–439. https://doi.org/10.1002/jcp.20033
Nurden AT (2007) Does ATP act through P2X 1 receptors to regulate platelet activation and thrombus formation?: Does ATP act through P2X1receptors? J Thromb Haemost 5:907–909. https://doi.org/10.1111/j.1538-7836.2007.02456.x
Koupenova M, Ravid K (2018) Biology of platelet purinergic receptors and implications for platelet heterogeneity. Front Pharmacol 9:37. https://doi.org/10.3389/fphar.2018.00037
Macfarlane DE, Mills DC (1975) The effects of ATP on platelets: evidence against the central role of released ADP in primary aggregation. Blood 46:309–320. https://doi.org/10.1182/blood.V46.3.309.bloodjournal463309
Packham MA, Mustard JF (2005) Platelet aggregation and adenosine diphosphate/adenosine triphosphate receptors: a historical perspective. Semin Thromb Hemost 31:129–138. https://doi.org/10.1055/s-2005-869518
Cekic C, Linden J (2016) Purinergic regulation of the immune system. Nat Rev Immunol 16:177–192. https://doi.org/10.1038/nri.2016.4
Junger WG (2011) Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol 11:201–212. https://doi.org/10.1038/nri2938
Allard B, Longhi MS, Robson SC, Stagg J (2017) The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev 276:121–144. https://doi.org/10.1111/imr.12528
Zhao H, Bo C, Kang Y, Li H (2017) What else can CD39 tell us? Front Immunol 8:727. https://doi.org/10.3389/fimmu.2017.00727
Vénéreau E, Ceriotti C, Bianchi ME (2015) DAMPs from cell death to new life. Front Immunol 6:422. https://doi.org/10.3389/fimmu.2015.00422
Marchi ED, Orioli E, Pegoraro A et al (2019) The P2X7 receptor modulates immune cells infiltration, ectonucleotidases expression and extracellular ATP levels in the tumor microenvironment. Oncogene 38:3636–3650. https://doi.org/10.1038/s41388-019-0684-y
Pandolfi F, Altamura S, Frosali S, Conti P (2016) Key role of DAMP in inflammation, cancer, and tissue repair. Clin Ther 38:1017–1028. https://doi.org/10.1016/j.clinthera.2016.02.028
Di Virgilio F, Dal Ben D, Sarti AC et al (2017) The P2X7 receptor in infection and inflammation. Immunity 47:15–31. https://doi.org/10.1016/j.immuni.2017.06.020
Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F (2017) The P2X7 receptor-interleukin-1 liaison. Front Pharmacol 8:123. https://doi.org/10.3389/fphar.2017.00123
Karunagaran D, Jinesh G (2008) TGF-β, Smads and cervical cancer. In: Jakowlew SB (ed) Cancer drug discovery and development: growth factor-β in Cancer therapy. Humana Press, Totowa, NJ, pp 33–49
Gao ZW, Wang HP, Dong K, Lin F, Wang X, Zhang HZ (2016) Adenosine inhibits migration, invasion and induces apoptosis of human cervical cancer cells. Neoplasma 63:201–207. https://doi.org/10.4149/204_150723N407
Merighi S, Mirandola P, Varani K et al (2003) A glance at adenosine receptors: novel target for antitumor therapy. Pharmacol Ther 100:31–48. https://doi.org/10.1016/S0163-7258(03)00084-6
Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J (2014) Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer 134:1466–1473. https://doi.org/10.1002/ijc.28456
Gorodeski GI (2009) P2X7-mediated chemoprevention of epithelial cancers. Expert Opin Ther Targets 13:1313–1332. https://doi.org/10.1517/14728220903277249
Fu W, McCormick T, Qi X, Luo L, Zhou L, Li X, Wang BC, Gibbons HE, Abdul-Karim FW, Gorodeski GI (2009) Activation of P2X7-mediated apoptosis inhibits DMBA/TPA-induced formation of skin papillomas and cancer in mice. BMC Cancer 9:114. https://doi.org/10.1186/1471-2407-9-114
de Lourdes M-GM, García-Rocha R, Morales-Ramírez O et al (2016) Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J Transl Med 14:302. https://doi.org/10.1186/s12967-016-1057-8
de Lourdes M-GM, López-Cisneros S, Gutiérrez-Serrano V et al (2019) HPV-16 infection is associated with a high content of CD39 and CD73 ectonucleotidases in cervical samples from patients with CIN-1. Mediat Inflamm 2019:4651627. https://doi.org/10.1155/2019/4651627
Gao Z, Wang H, Lin F, Wang X, Long M, Zhang HZ, Dong K (2017) CD73 promotes proliferation and migration of human cervical cancer cells independent of its enzyme activity. BMC Cancer 17:1–8. https://doi.org/10.1186/s12885-017-3128-5
Ghalamfarsa G, Kazemi MH, Raoofi Mohseni S, Masjedi A, Hojjat-Farsangi M, Azizi G, Yousefi M, Jadidi-Niaragh F (2019) CD73 as a potential opportunity for cancer immunotherapy. Expert Opin Ther Targets 23:127–142. https://doi.org/10.1080/14728222.2019.1559829
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
MS Pfaffenzeller declares that she has no conflict of interest. MLM Franciosi declares that she has no conflict of interest. AM Cardoso declares that she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pfaffenzeller, M.S., Franciosi, M.L.M. & Cardoso, A.M. Purinergic signaling and tumor microenvironment in cervical Cancer. Purinergic Signalling 16, 123–135 (2020). https://doi.org/10.1007/s11302-020-09693-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-020-09693-3