Abstract
Background
Lipopolysaccharide (LPS)-induced acute kidney injury (AKI) is associated with an abnormal immune response. Accumulating evidence has demonstrated that aquaporin 1 (AQP1) prevents kidney tissue injury in LPS-induced AKI by mediating immune response. However, the underlying mechanisms remain obscure. Macrophages as immune cells with multiple phenotypes are important mediators in tissue homeostasis and host defense. We propose that macrophage polarization is implicated in AQP1-mediated immune response.
Methods
Herein we established sepsis-induced AKI model rats through intraperitoneal injection of LPS into Wistar rats to reveal immune mechanism of damage. We also used LPS-induced mouse RAW264.7 cells to elucidate the molecular mechanism of macropage polarization.
Results
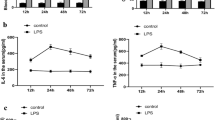
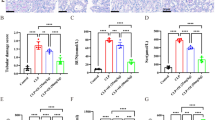
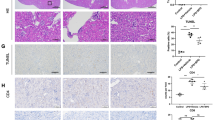
Histopathology showed that renal tubular epithelial cells in the model group were swollen, inflammatory exudation was obvious and the inflammatory factors, interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) were increased. Western blotting showed PI3K was upregulated in the model group. Serum creatinine and urea nitrogen increased after LPS injection. Renal AQP1 mRNA is downregulated and serum AQP1 protein increased first and then decreased in LPS-induced AKI rats. M2 macrophage markers (Arg-1, CD206) were increased in repair stage. In addition, treatment of murine macrophages (RAW264.7) with AQP1 siRNA resulted in decreased PI3K activation and M2 polarization, but increased IL-6 and TNF-α. Moreover, inhibiting PI3K with wortmannin imitated the results of AQP1 silencing.
Conclusions
Macrophage M2 polarization is likely the cellular mechanism underlying the anti-AKI property of AQP1, and PI3K activation is involved in the AQP1-induced M2 phenotype switch.








Similar content being viewed by others
References
Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–91.
Li C, Wu J, Li Y, Xing G. Cytoprotective effect of heat shock protein 27 against Lipopolysaccharide-induced apoptosis of renal epithelial HK-2 cell. Cell Physiol Biothem. 2017;41:2211–20.
Rivers EP, Coba V, Whitmill M. Early goal-directed in severe sepsis and septic shock: a contemporary review of the literature. Curr Opin Anaesthesiol. 2008;21:128–40.
Zhong F, Chen H, Han L, Jin Y, Wang W. Curcumin attenuates lipopolysaccharide-induced renal inflammation. Biol Pharm Bull. 2011;34:226–32.
Monzani E, Bazzotti R, Perego C, La Porta CA. AQP1 is not only a water channel: it contributes to cell migration through Lin7/beta-catenin. PLoS ONE. 2009;4:e6167.
Stock C, Schwab A. Ion channels and transporters in metastasis. Biochim Biophys Acta. 2015;1848:2638–46.
Pelagalli A, Nardelli A, Fontanella R, et al. Inhibition of AQP1 hampers osteosarcoma and hepatocellular carcinoma progression mediated by bone marrow-derived mesenchymal stem cells. Int J Mol Sci. 2016;17(7):1102.
Jin Y, Yu G, Peng P, Zhang Y, Xin X. Down-regulated expression of AQP5 on lung in rat DIC model induced by LPS and its effect on the development of pulmonary edema. Pulm Pharmacol Ther. 2013;26:661–5.
Jiang YX, Dai ZL, Zhang XP, Zhao W, Huang Q, Gao LK. Dexmedetomidine alleviates pulmonary edema by upregulating AQP1 and AQP5 expression in rats with acute lung injury induced by lipopolysaccharide. Huazhong Univ Sci Technol Med Sci. 2015;35:684–8.
Nielsen S, Kwon TH, Frokiaer J, Agre P. Regulation and dysregulation of aquaporins in water balance disorders. Intern Med. 2007;261:53–64.
Marrone J, Danielli M, Gaspari CI, Marinelli RA. Adenovirus-mediated human aquaporin-1 expression in hepatocytes improves lipopolysaccharide-induced cholestasis. IUBMB Life. 2017;69:978–84.
Li J, Zhang M, Mao Y, Li Y, Zhang X, Peng X, Yu F. The potential role of aquaporin1 on aristolochic acid I induced epithelial mesenchymal transition on HK-2 cells. Cell Physiol. 2018;233:4919–25.
Tyteca D, Nishino T, Debaix H, Van Der Smissen P, N’kuli F, Hoffmann D, et al. Regulation of macrophage motility by the water channel aquaporin-1: crucial role of M0/M2 phenotype switch. PLoS ONE. 2015;10(2):e0117398.
Dong XY, Liu CM, Li BH, et al. Correlation between urinary aquaporin level and inflammatory factors in disseminated intravascular coagulation with acute non-oliguric renal injury. Chin J Pract Diagn Treat. 2019;33(3):216–20.
Wang YD, Zhang WZ, Yu GZ, Liu Q, Jin YY. Cytoprotective effect of aquaporin1 against lipopolysaccharide-induced apoptosis and inflammation of renal epithelial HK-2 cells. Exp Ther Med. 2018;15:4243–52.
Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int. 2006;69:1996–2002.
Martinez FO. Regulators of macrophage activation. Eur J Immunol. 2011;41(6):1531–4.
Martinez FO, Sica A, Mantovani A, et al. Macrophage activation and polarization. Front Biosci. 2008;13:453.
Sun K, He SB, Qu JG, et al. IRF5 regulates lung macrophages M2 polarization during severe acute pancreatitis in vitro. World J Gastroenterol. 2016;22(42):9368–77.
Watanabe T, Kudo M, Strober W. Immunopathogenesis of pancreatitis. Mucosal Immunol. 2017;10(2):283–98.
Humphreys BD. Targeting endogenous repair pathways after AKI. J Am Soc Nephrol. 2016;27:990–7.
Huen SC, Cantley LG. Macrophages in renal injury and repair. Annu Rev Physiol. 2017;79:449–69.
Zhou Y, Tu C, Zhao Y, et al. Placental growth factor enhances angiogenesis in human intestinal microvascular endothelial cells via PI3K/Akt pathway: potential implications of inflammation bowel disease. Biochem Biophys Res Commun. 2016;470(4):967–74.
Burke JE, Williams RL. Synergy in activating class PI3Ks. Trends Biochem Sci. 2015;40(2):88–100.
Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol JASN. 2011;22:999–1006.
Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Investig. 2009;119:2868–78.
Zhang Y, Chen J, Zhang K, et al. Inflammation and oxidative stress are associated with the prevalence of high ankle-brachial index in metabolic syndrome patients without chronic renal failure. Int J Med Sci. 2013;10(2):183–90.
Qin H, Holdbrooks AT, Liu Y, et al. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189:3439–48.
Liu X, Mu GH, Song C, Zhou L, et al. Role of M2 macrophages in sepsis-induced acute kidney injury. Shock. 2018;50(2):233–9.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61.
Sonoda H, Yokota-Ikeda N, Oshikawa S, Kanno Y. Decreased abundance of urinary exosomal aquaporin-1 in renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F1006–F10161016.
Tyteca D, Nishino T, Debaix H, et al. Regulation of macrophage motility by the water channel aquaporin-1: crucial role of M0/M2 phenotype switch. PLoS ONE. 2015;10(2):e0117398.
Kishore BK, Krane CM, Di Iulio D, et al. Expression of renal aquaporins 1, 2, and 3 in a rat model of cisplatin-induced polyuria. Int Soc Nephrol. 2000;58:701–11.
Asvapromtada S, Sonoda H, Kinouchi M, et al. Characterization of urinary exosomal release of aquaporin-1 and -2 after renal ischemia-reperfusion in rats. Am J Physiol Renal Physiol. 2018;314:F584–F601.
Nakhoul NL, Davis BA, Romero MF, Boron WF. Effect of expressing the water channel aquaporin-1 on the CO2 permeability of Xenopus oocytes. Am J Physiol. 1998;274:C543–8.
Echevarría M, Muñoz-Cabello AM, Sánchez-Silva R, et al. Development of cytosolic hypoxia and hypoxia-inducible factor stabilization are facilitated by aquaporin-1 expression. J Biol Chem. 2007;282(41):30207–155.
Jiao G, Li E, Yu R. Decreased expression of AQP1 and AQP5 in acute injured lungs in rats. Chin Med J (Engl). 2002;15:963–7.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96.
Dasgupta P, Keegan AD. Contribution of alternatively activated macrophages to allergic lung inflammation: a tale of mice and men. Innate Immun. 2012;4:478–88.
Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, et al. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;9:480–98.
Acknowledgements
We thank Michal Bell, Ph.D., from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
This study was supported by the Natural Youth Science Foundation of China (grant no. 81501825) and Youth Science Foundation of Heilongjiang Province of China Grant (grant no. QC2012C035).
Author information
Authors and Affiliations
Contributions
CL and BL performed the experiments. XD, LX, GS and KT analyzed the data. YJ made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, acquisition of funding. All authors gave their final approval of the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Ethics statement
The study protocol was approved by the Animal Care and Use Committee of Harbin Medical University (No: 2019014), and the experimental procedures were in accordance with the recommendations of the Guide for the care and use of Laboratory Animals, published by the US National Institutes of Health (NIH Publication no. 85-23. Revised 196).
Additional information
Responsible Editor: John Di Battista.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, C., Li, B., Tang, K. et al. Aquaporin 1 alleviates acute kidney injury via PI3K-mediated macrophage M2 polarization. Inflamm. Res. 69, 509–521 (2020). https://doi.org/10.1007/s00011-020-01334-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-020-01334-0