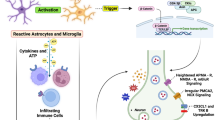
Abstract
The therapeutic effect of electroacupuncture (EA) on inflammatory pain has been well recognized clinically, but the mechanism is unclear. Interleukin-10 (IL-10), which is produced by regulatory T (Treg) cell, is a key anti-inflammatory cytokine for relieving inflammatory pain. Therefore, the aim of this study is to investigate whether EA could inhibit CFA-induced pain and attenuate inflammation progression by regulating the activation of immunocyte and inducing the expression of IL-10. In this study, mice were treated with EA (2/100 Hz, 2 mA) for five consecutive days after 1 day of CFA injection. The behavioral tests were measured and analyzed after the daily EA treatment; then, hind paw, spinal cord, and spleen tissues were prepared for assessment. The results showed that EA treatment significantly increased the mechanical threshold and thermal latency after CFA injection and boosted the expression of IL-10 in paw and spinal cord tissues. EA treatment promoted Treg cells; suppressed macrophage and neutrophils cells; reduced the expression of IL-1β, NLRP3, and TNF-α; and ultimately relieved inflammatory pain. The findings suggested that the analgesic and anti-inflammatory effect of EA treatment could be partially associated with suppression of pro-inflammatory cytokines mediated by induction of IL-10.






Similar content being viewed by others
References
Pedersen, J.L. 2000. Inflammatory pain in experimental burns in man. Danish Medical Bulletin 47: 168–195.
Held, M., F. Karl, E. Vlckova, A. Rajdova, F. Escolano-Lozano, C. Stetter, R. Bharti, K.U. Förstner, M. Leinders, L. Dušek, F. Birklein, J. Bednarik, C. Sommer, and N. Üçeyler. 2019. Sensory profiles and immune related expression patterns of patients with and without neuropathic pain after peripheral nerve lesion. Pain 160: 2316–2327.
Pinho-Ribeiro, F.A., W.A. Verri Jr., and I.M. Chiu. 2017. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends in Immunology 38: 5–19.
Baral, P., S. Udit, and I.M. Chiu. 2019. Pain and immunity: implications for host defence. Nature Reviews Immunology 19: 433–447.
Mueller, M., C. Leonhard, K. Wacker, E.B. Ringelstein, M. Okabe, W.F. Hickey, and R. Kiefer. 2003. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Laboratory Investigation; a journal of technical methods and pathology 83: 175–185.
Perrin, F.E., S. Lacroix, M. Aviles-Trigueros, and S. David. 2005. Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain : a journal of neurology 128: 854–866.
Dunster, J.L. 2016. The macrophage and its role in inflammation and tissue repair: mathematical and systems biology approaches. Wiley Interdisciplinary Reviews. Systems Biology and Medicine 8: 87–99.
Zhu, L., C. Jones, and G. Zhang. 2018. The role of phospholipase C signaling in macrophage-mediated inflammatory response. Journal of Immunology Research 2018: 5201759.
Paulsen, O., B. Laird, N. Aass, T. Lea, P. Fayers, S. Kaasa, and P. Klepstad. 2017. The relationship between pro-inflammatory cytokines and pain, appetite and fatigue in patients with advanced cancer. PLoS One 12: e0177620.
Purdy, M., M. Kokki, M. Anttila, S. Aspinen, P. Juvonen, R. Korhonen, T. Selander, H. Kokki, and M. Eskelinen. 2016. Does the rectus sheath block analgesia reduce the inflammatory response biomarkers' IL-1ra, IL-6, IL-8, IL-10 and IL-1beta concentrations following surgery? A randomized clinical trial of patients with cancer and benign disease. Anticancer Research 36: 3005–3011.
Ip, W.K.E., N. Hoshi, D.S. Shouval, S. Snapper, and R. Medzhitov. 2017. Anti-inflammatory effect of IL-10 mediated by metabolic reprogramming of macrophages. Science 356: 513–519.
Duffy, S.S., B.A. Keating, C.J. Perera, J.G. Lees, R.S. Tonkin, P.G.S. Makker, P. Carrive, O. Butovsky, and G. Moalem-Taylor. 2019. Regulatory T cells and their derived cytokine, interleukin-35, reduce pain in experimental autoimmune encephalomyelitis. The Journal of neuroscience : the official journal of the Society for Neuroscience 39: 2326–2346.
Zazueta-Favela, D., L. Donis-Maturano, A.F. Licea-Navarro, J. Bernaldez-Sarabia, K.W.L. Dan, J.M. Cota-Arce, G. Escobedo, and M.A. De Leon-Nava. 2019. Marine peptides as immunomodulators: Californiconus californicus-derived synthetic conotoxins induce IL-10 production by regulatory T cells (CD4(+)Foxp3(+)). Immunopharmacology and Immunotoxicology 41: 463–468.
Azizzadeh, F., J. Mahmoodi, S. Sadigh-Eteghad, F. Farajdokht, and G. Mohaddes. 2017. Ghrelin exerts analgesic effects through modulation of IL-10 and TGF-beta levels in a rat model of inflammatory pain. Iranian Biomedical Journal 21: 114–119.
Kwon, J.Y., S.H. Lee, H.S. Na, K. Jung, J. Choi, K.H. Cho, C.Y. Lee, et al. 2018. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Scientific Reports 8: 13832.
Milligan, E.D., K.R. Penzkover, R.G. Soderquist, and M.J. Mahoney. 2012. Spinal interleukin-10 therapy to treat peripheral neuropathic pain. Neuromodulation : journal of the International Neuromodulation Society 15: 520–526.
Plunkett, J.A., C.G. Yu, J.M. Easton, J.R. Bethea, and R.P. Yezierski. 2001. Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Experimental Neurology 168: 144–154.
Vanderwall, A.G., S. Noor, M.S. Sun, J.E. Sanchez, X.O. Yang, L.L. Jantzie, N. Mellios, and E.D. Milligan. 2018. Effects of spinal non-viral interleukin-10 gene therapy formulated with d-mannose in neuropathic interleukin-10 deficient mice: behavioral characterization, mRNA and protein analysis in pain relevant tissues. Brain, Behavior, and Immunity 69: 91–112.
Park, J.Y., and U. Namgung. 2018. Electroacupuncture therapy in inflammation regulation: current perspectives. Journal of Inflammation Research 11: 227–237.
Vickers, A.J., A.M. Cronin, A.C. Maschino, G. Lewith, H. MacPherson, N.E. Foster, K.J. Sherman, et al. 2012. Acupuncture for chronic pain: individual patient data meta-analysis. Archives of Internal Medicine 172: 1444–1453.
Vickers, A.J., E.A. Vertosick, G. Lewith, H. MacPherson, N.E. Foster, K.J. Sherman, D. Irnich, C.M. Witt, K. Linde, and Acupuncture Trialists' Collaboration. 2018. Acupuncture for chronic pain: update of an individual patient data meta-analysis. The Journal of Pain : official journal of the American Pain Society 19: 455–474.
Kong, J., D.T. Fufa, A.J. Gerber, I.S. Rosman, M.G. Vangel, R.H. Gracely, and R.L. Gollub. 2005. Psychophysical outcomes from a randomized pilot study of manual, electro, and sham acupuncture treatment on experimentally induced thermal pain. The Journal of Pain : official journal of the American Pain Society 6: 55–64.
Liu, Z., Y. Liu, H. Xu, L. He, Y. Chen, L. Fu, N. Li, Y. Lu, T. Su, J. Sun, J. Wang, Z. Yue, W. Zhang, J. Zhao, Z. Zhou, J. Wu, K. Zhou, Y. Ai, J. Zhou, R. Pang, Y. Wang, Z. Qin, S. Yan, H. Li, L. Luo, and B. Liu. 2017. Effect of electroacupuncture on urinary leakage among women with stress urinary incontinence: a randomized clinical trial. JAMA 317: 2493–2501.
Meng, J.B., Y.N. Jiao, X.J. Xu, Z.Z. Lai, G. Zhang, C.L. Ji, and M.H. Hu. 2018. Electro-acupuncture attenuates inflammatory responses and intraabdominal pressure in septic patients: a randomized controlled trial. Medicine 97: e0555.
Meng, J.B., Y.N. Jiao, G. Zhang, X.J. Xu, C.L. Ji, M.H. Hu, Z.Z. Lai, and M. Zhang. 2018. Electroacupuncture improves intestinal dysfunction in septic patients: a randomised controlled trial. BioMed Research International 2018: 8293594.
Wang, Z., T. Chen, M. Long, L. Chen, L. Wang, N. Yin, and Z. Chen. 2017. Electro-acupuncture at Acupoint ST36 ameliorates inflammation and regulates Th1/Th2 balance in delayed-type hypersensitivity. Inflammation 40: 422–434.
Gao, F., H.C. Xiang, H.P. Li, M. Jia, X.L. Pan, H.L. Pan, and M. Li. 2018. Electroacupuncture inhibits NLRP3 inflammasome activation through CB2 receptors in inflammatory pain. Brain, Behavior, and Immunity 67: 91–100.
Su, T.F., Y.Q. Zhao, L.H. Zhang, M. Peng, C.H. Wu, L. Pei, B. Tian, et al. 2012. Electroacupuncture reduces the expression of proinflammatory cytokines in inflamed skin tissues through activation of cannabinoid CB2 receptors. European Journal of Pain 16: 624–635.
da Silva, M.D., F. Bobinski, K.L. Sato, S.J. Kolker, K.A. Sluka, and A.R. Santos. 2015. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Molecular Neurobiology 51: 19–31.
Qin, F., H. Zhang, A. Liu, Q. Wang, Q. Sun, S. Lu, Q. Li, et al. 2019. Analgesic effect of Zanthoxylum nitidum extract in inflammatory pain models through targeting of ERK and NF-kappaB signaling. Frontiers in Pharmacology 10: 359.
Jeong, J.H., S.J. Moon, J.Y. Jhun, E.J. Yang, M.L. Cho, and J.K. Min. 2015. Eupatilin exerts antinociceptive and chondroprotective properties in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. PLoS One 10: e0130882.
Slotkin, J.R., J.K. Ness, K.M. Snyder, A.A. Skiles, E.J. Woodard, T. O'Shea, R.T. Layer, et al. 2016. Sustained local release of methylprednisolone from a thiol-acrylate poly(ethylene glycol) hydrogel for treating chronic compressive radicular pain. Spine, 41: E441–E448.
Xiang, H.C., L.X. Lin, X.F. Hu, H. Zhu, H.P. Li, R.Y. Zhang, L. Hu, et al. 2019. AMPK activation attenuates inflammatory pain through inhibiting NF-kappaB activation and IL-1beta expression. Journal of Neuroinflammation 16: 34.
Chen, G., Y.H. Kim, H. Li, H. Luo, D.L. Liu, Z.J. Zhang, M. Lay, et al. 2017. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nature Neuroscience 20: 917–926.
Hussein, S.Z., K. Mohd Yusoff, S. Makpol, and Y.A. Mohd Yusof. 2013. Gelam honey attenuates carrageenan-induced rat paw inflammation via NF-kappaB pathway. PLoS One 8: e72365.
Rose, S., A. Misharin, and H. Perlman. 2012. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry Part A : the journal of the International Society for Analytical Cytology 81: 343–350.
McCarson, K.E. 2015. Models of inflammation: Carrageenan- or complete Freund’s adjuvant (CFA)-induced edema and hypersensitivity in the rat. Current Protocols in Pharmacology 70: 5 4–1-9.
Fan, X.C., S. Fu, F.Y. Liu, S. Cui, M. Yi, and Y. Wan. 2018. Hypersensitivity of prelimbic cortex neurons contributes to aggravated nociceptive responses in rats with experience of chronic inflammatory pain. Frontiers in Molecular Neuroscience 11: 85.
Zhou, Y., Y. Suzuki, K. Uchida, and M. Tominaga. 2013. Identification of a splice variant of mouse TRPA1 that regulates TRPA1 activity. Nature Communications 4: 2399.
Fang, J.Q., J.Y. Du, J.F. Fang, T. Xiao, X.Q. Le, N.F. Pan, J. Yu, and B.Y. Liu. 2018. Parameter-specific analgesic effects of electroacupuncture mediated by degree of regulation TRPV1 and P2X3 in inflammatory pain in rats. Life Sciences 200: 69–80.
Kosek, E., M. Cohen, R. Baron, G.F. Gebhart, J.A. Mico, A.S. Rice, W. Rief, and A.K. Sluka. 2016. Do we need a third mechanistic descriptor for chronic pain states? Pain 157: 1382–1386.
Vardeh, D., R.J. Mannion, and C.J. Woolf. 2016. Toward a mechanism-based approach to pain diagnosis. The Journal of Pain : official journal of the American Pain Society 17: T50–T69.
Planells-Cases, R., N. Garcia-Sanz, C. Morenilla-Palao, and A. Ferrer-Montiel. 2015. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflügers Archiv / European Journal of Physiology 451: 151–159.
Zhang, J.H., and Y.G. Huang. 2006. The immune system: a new look at pain. Chinese Medical Journal 119: 930–938.
Ren, K., and R. Torres. 2009. Role of interleukin-1beta during pain and inflammation. Brain Research Reviews 60: 57–64.
Parameswaran, N., and S. Patial. 2010. Tumor necrosis factor-alpha signaling in macrophages. Critical Reviews in Eukaryotic Gene Expression 20: 87–103.
Zhang, J.M., and J. An. 2007. Cytokines, inflammation, and pain. International Anesthesiology Clinics 45: 27–37.
Abderrazak, A., T. Syrovets, D. Couchie, K. El Hadri, B. Friguet, T. Simmet, and M. Rouis. 2015. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biology 4: 296–307.
Davis, B.K., H. Wen, and J.P. Ting. 2011. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annual Review of Immunology 29: 707–735.
Marchetti, C. 2019. The NLRP3 inflammasome as pharmacological target. Journal of Cardiovascular Pharmacology 74: 285–296.
Huang, D., M. Chen, Z. Wang, L. Hou, and W. Yu. 2019. Electroacupuncture pretreatment attenuates inflammatory lung injury after cardiopulmonary bypass by suppressing NLRP3 inflammasome activation in rats. Inflammation 42: 895–903.
Penatti, A., F. Facciotti, R. De Matteis, P. Larghi, M. Paroni, A. Murgo, O. De Lucia, et al. 2017. Differences in serum and synovial CD4+ T cells and cytokine profiles to stratify patients with inflammatory osteoarthritis and rheumatoid arthritis. Arthritis Research & Therapy 19: 103.
Jin, R.M., J. Warunek, and E.A. Wohlfert. 2018. Therapeutic administration of IL-10 and amphiregulin alleviates chronic skeletal muscle inflammation and damage induced by infection. ImmunoHorizons 2: 142–154.
Li, S., J. Wan, W. Anderson, H. Sun, H. Zhang, X. Peng, Z. Yu, et al. 2016. Downregulation of IL-10 secretion by Treg cells in osteoarthritis is associated with a reduction in Tim-3 expression. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie 79: 159–165.
Sharma, A., and D. Rudra. 2019. Regulatory T cells as therapeutic targets and mediators. International Reviews of Immunology 38: 183–203.
Tanaka, A., and S. Sakaguchi. 2017. Regulatory T cells in cancer immunotherapy. Cell Research 27: 109–118.
Cunha, T.M., W.A. Verri Jr., I.R. Schivo, M.H. Napimoga, C.A. Parada, S. Poole, M.M. Teixeira, S.H. Ferreira, and F.Q. Cunha. 2008. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. Journal of Leukocyte Biology 83: 824–832.
Inglis, J.J., A. Nissim, D.M. Lees, S.P. Hunt, Y. Chernajovsky, and B.L. Kidd. 2005. The differential contribution of tumour necrosis factor to thermal and mechanical hyperalgesia during chronic inflammation. Arthritis Research & Therapy 7: R807–R816.
Segond von Banchet, G., M.K. Boettger, N. Fischer, M. Gajda, R. Brauer, and H.G. Schaible. 2009. Experimental arthritis causes tumor necrosis factor-alpha-dependent infiltration of macrophages into rat dorsal root ganglia which correlates with pain-related behavior. Pain 145: 151–159.
Ghasemlou, N., I.M. Chiu, J.P. Julien, and C.J. Woolf. 2015. CD11b+Ly6G- myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proceedings of the National Academy of Sciences of the United States of America 112: E6808–E6817.
Kobayashi, Y., N. Kiguchi, Y. Fukazawa, F. Saika, T. Maeda, and S. Kishioka. 2015. Macrophage-T cell interactions mediate neuropathic pain through the glucocorticoid-induced tumor necrosis factor ligand system. The Journal of Biological Chemistry 290: 12603–12613.
Schuh, C.D., S. Pierre, A. Weigert, B. Weichand, K. Altenrath, Y. Schreiber, N. Ferreiros, D.D. Zhang, J. Suo, E.M. Treutlein, M. Henke, H. Kunkel, M. Grez, R. Nüsing, B. Brüne, G. Geisslinger, and K. Scholich. 2014. Prostacyclin mediates neuropathic pain through interleukin 1beta-expressing resident macrophages. Pain 155: 545–555.
Shutov, L.P., C.A. Warwick, X. Shi, A. Gnanasekaran, A.J. Shepherd, D.P. Mohapatra, T.M. Woodruff, J.D. Clark, and Y.M. Usachev. 2016. The complement system component C5a produces thermal Hyperalgesia via macrophage-to-nociceptor signaling that requires NGF and TRPV1. The Journal of neuroscience : the official journal of the Society for Neuroscience 36: 5055–5070.
Kim, C.F., and G. Moalem-Taylor. 2011. Interleukin-17 contributes to neuroinflammation and neuropathic pain following peripheral nerve injury in mice. The Journal of Pain : official journal of the American Pain Society 12: 370–383.
Aich, A., L.B. Afrin, and K. Gupta. 2015. Mast cell-mediated mechanisms of nociception. International Journal of Molecular Sciences 16: 29069–29092.
Chatterjea, D., and T. Martinov. 2015. Mast cells: versatile gatekeepers of pain. Molecular Immunology 63: 38–44.
Tanaka, T., M. Narazaki, and T. Kishimoto. 2014. IL-6 in inflammation, immunity, and disease. Cold Spring Harbor Perspectives in Biology 6: a016295.
Acknowledgments
We thank Prof. Ye Yang of Nanjing University of Chinese Medicine for his help with the operation on flow cytometry.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81774210, 81574062), “Six Major Talent Summit” of Jiangsu Province (YY-033), Jiangsu Province “333 High-level Talents Cultivating Project” (2016), “Qing Lan Project” of Jiangsu Province and A Project Funded by the Nursing Priority Academic Program Development of Jiangsu Higher Education Institutions (2019YSHL046). The funders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: SFL ZGL MLY. Performed the experiments: MLY RDW SFL JMW TZ FFQ SPF. Analyzed the data: MLY SFL RDW. Wrote the paper: SFL MLY ZGL. All authors have read and agreed with the manuscript.
Corresponding authors
Ethics declarations
All procedures were strictly carried out in accordance with the guidelines of the NIH Animal Care and Use Committee, and the study was approved by the Institutional Animal Care and Use Committee of Nanjing University of Chinese Medicine.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, Ml., Wei, Rd., Zhang, T. et al. Electroacupuncture Relieves Pain and Attenuates Inflammation Progression Through Inducing IL-10 Production in CFA-Induced Mice. Inflammation 43, 1233–1245 (2020). https://doi.org/10.1007/s10753-020-01203-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-020-01203-2