Abstract
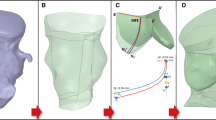
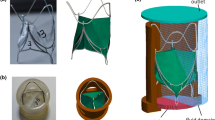
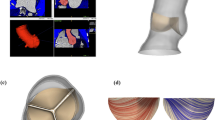
Transcatheter aortic valve replacement (TAVR) is a minimally invasive procedure that provides an effective alternative to open-heart surgical valve replacement for treating advanced calcific aortic valve disease patients. However, complications, such as valve durability, device migration, paravalvular leakage (PVL), and thrombogenicity may lead to increased overall post-TAVR morbidity and mortality. A series of numerical studies involving a self-expandable TAVR valve were performed to evaluate these complications. Structural studies were performed with finite element (FE) analysis, followed by computational fluid dynamics (CFD) simulations, and fluid–structure interaction (FSI) analysis. The FE analysis was utilized to study the effect of TAVR valve implantation depth on valve anchorage in the Living Heart Human Model, which is capable of simulating beating heart during repeated cardiac cycles. The TAVR deployment cases where no valve migration was observed were then used to calculate the post-deployment thrombogenic potential via CFD simulations. FSI analysis followed to further assess the post-deployment TAVR hemodynamic performance for different implantation depths. The deployed valves PVL, geometric and effective orifice areas, and the leaflets structural and flow stress magnitudes were compared to determine the device optimal landing zone. The combined structural and hemodynamic analysis indicated that with the TAVR valve deployed at an aft ventricle position an optimal performance was achieved in the specific anatomy studied. Given the TAVR’s rapid expansion to younger lower-risk patients, the comprehensive numerical methodology proposed here can potentially be used as a predictive tool for both procedural planning and valve design optimization to minimize the reported complications.







Similar content being viewed by others
References
Abbasi M et al (2019) A non-invasive material characterization framework for bioprosthetic heart valves. Ann Biomed Eng 47:97–112
Bailey J, Curzen N, Bressloff NW (2016) Assessing the impact of including leaflets in the simulation of TAVI deployment into a patient-specific aortic root. Comput Methods Biomech Biomed Eng 19:733–744
Baillargeon B et al (2014) The Living Heart Project: a robust and integrative simulator for human heart function. Eur J Mech A Solids 48:38–47
Bianchi M et al (2016) Effect of balloon-expandable transcatheter aortic valve replacement positioning: a patient-specific numerical model. Artif Organs 40(12):E292–E304
Bianchi M et al (2019) Patient-specific simulation of transcatheter aortic valve replacement: impact of deployment options on paravalvular leakage. Biomech Model Mechanobiol 18(2):435–451
Bidar E et al (2019) Postimplant biological aortic prosthesis degeneration: challenges in transcatheter valve implants. Eur J Cardiothorac Surg 55(2):191–200
Bosmans B et al (2016) A validated methodology for patient specific computational modeling of self-expandable transcatheter aortic valve implantation. J Biomech 49(13):2824–2830
Caballero A et al (2017) Evaluation of transcatheter heart valve biomaterials: biomechanical characterization of bovine and porcine pericardium. J Mech Behav Biomed Mater 75:486–494
Cao K, Sucosky P (2017) Computational comparison of regional stress and deformation characteristics in tricuspid and bicuspid aortic valve leaflets. Int J Numer Method Biomed Eng 33(3):e02798
Chandra S, Rajamannan N, Sucosky P (2012) Computational assessment of bicuspid aortic valve wall-shear stress: implications for calcific aortic valve disease. Biomech Model Mechanobiol 11(7):1085–1096
Claiborne TE et al (2013) In vitro evaluation of a novel hemodynamically optimized trileaflet polymeric prosthetic heart valve. J Biomech Eng 135(2):021021
Dasi LP et al (2009) Fluid mechanics of artificial heart valves. Clin Exp Pharmacol Physiol 36(2):225–237
de Jaegere P et al (2016) Patient-specific computer modeling to predict aortic regurgitation after transcatheter aortic valve replacement. JACC Cardiovasc Interv 9(5):508–512
FDA (2019) FDA expands indication for several transcatheter heart valves to patients at low risk for death or major complications associated with open-heart surgery
Finotello A, Morganti S, Auricchio F (2017) Finite element analysis of TAVI: impact of native aortic root computational modeling strategies on simulation outcomes. Med Eng Phys 47:2–12
Franzoni I et al (2013) Comparison of incidence and predictors of left bundle branch block after transcatheter aortic valve implantation using the CoreValve versus the Edwards valve. Am J Cardiol 112(4):554–559
Fuchs A et al (2018) Commissural alignment of bioprosthetic aortic valve and native aortic valve following surgical and transcatheter aortic valve replacement and its impact on valvular function and coronary filling. JACC Cardiovasc Interv 11:1733–1743
Garcia D, Kadem L (2006) What do you mean by aortic valve area: geometric orifice area, effective orifice area, or gorlin area? J Heart Valve Dis 15(5):601–608
Geisbüsch S et al (2010) Incidence and management of CoreValve dislocation during transcatheter aortic valve implantation. Circ Cardiovasc Interv 3(6):531–536
Ghosh R et al (2018) Comparative fluid–structure interaction analysis of polymeric transcatheter and surgical aortic valves’ hemodynamics and structural mechanics. J Biomech Eng. https://doi.org/10.1115/1.4040600
Giblett JP et al (2019) Percutaneous management of paravalvular leaks. Nat Rev Cardiol 16:275–285
Gilbert ON et al (2018) Comparison of paravalvular aortic leak characteristics in the Medtronic CoreValve versus Edwards Sapien Valve: paravalvular aortic leak characteristics. Catheter Cardiovasc Interv 92:972–980
Gilmanov A, Sotiropoulos F (2015) Comparative hemodynamics in an aorta with bicuspid and trileaflet valves. Theoret Comput Fluid Dyn 30(1–2):67–85
Goel K et al (2018) Relationship between procedural characteristics and cerebrovascular events after transcatheter aortic valve replacement. Open Heart 5(2):e000816
Hahn RT et al (2018) Comprehensive echocardiographic assessment of normal transcatheter valve function. JACC Cardiovasc Imaging 12:25–34
Kandail HS et al (2018) Impact of annular and supra-annular CoreValve deployment locations on aortic and coronary artery hemodynamics. J Mech Behav Biomed Mater 86:131–142
Luraghi G et al (2019) On the modeling of patient-specific transcatheter aortic valve replacement: a fluid–structure interaction approach. Cardiovasc Eng Technol 10:437–455
Mao W et al (2018) Numerical parametric study of paravalvular leak following a transcatheter aortic valve deployment into a patient-specific aortic root. J Biomech Eng 140(10):101007-101007-11
Marom G, Bluestein D (2016) Lagrangian methods for blood damage estimation in cardiovascular devices—how numerical implementation affects the results. Expert Rev Med Devices 13(2):113–122
Marom G et al (2014) Numerical model of full-cardiac cycle hemodynamics in a total artificial heart and the effect of its size on platelet activation. J Cardiovas Transl Res 7(9):788–796
Marom G et al (2016) Electro-mechanical modeling of transcatheter aortic valve deployment in the Simulia Living Heart Human Model. In: NAFEMS: multiphysics simulation, Copenhagen, Denmark
Midha PA et al (2016) Valve type, size, and deployment location affect hemodynamics in an in vitro valve-in-valve model. JACC Cardiovasc Interv 9(15):1618–1628
Midha PA et al (2017) The fluid mechanics of transcatheter heart valve leaflet thrombosis in the neosinus. Circulation 136(17):1598–1609
Morganti S et al (2016) Prediction of patient-specific post-operative outcomes of TAVI procedure: the impact of the positioning strategy on valve performance. J Biomech 49:2513–2519
Petronio AS et al (2015) Optimal implantation depth and adherence to guidelines on permanent pacing to improve the results of transcatheter aortic valve replacement with the Medtronic CoreValve System: The CoreValve Prospective, International, Post-Market ADVANCE-II Study. JACC Cardiovasc Interv 8(6):837–846
Pibarot P et al (2015) Assessment of paravalvular regurgitation following TAVR: a proposal of unifying grading scheme. JACC Cardiovasc Imaging 8(3):340–360
Pott D et al (2015) Development of a transcatheter tricuspid valve prosthesis through steps of iterative optimization and finite element analysis. Artif Organs 39:903–915
Rocatello G et al (2018) Patient-specific computer simulation to elucidate the role of contact pressure in the development of new conduction abnormalities after catheter-based implantation of a self-expanding aortic valve. Circ Cardiovasc Interv 11(2):e005344
Rotman OM et al (2018) Principles of TAVR valve design, modelling, and testing. Expert Rev Med Devices 15(11):771–791
Schultz C et al (2016) Patient-specific image-based computer simulation for theprediction of valve morphology and calcium displacement after TAVI with the Medtronic CoreValve and the Edwards SAPIEN valve. EuroIntervention 11(9):1044–1052
Sedrakyan A, Dhruva SS, Shuhaiber J (2016) Transcatheter aortic valve replacement in younger individuals. JAMA Intern Med 177(2):2
Sodhani D et al (2018) Fluid–structure interaction simulation of artificial textile reinforced aortic heart valve: validation with an in vitro test. J Biomech 78:52–69
Tanaka Y et al (2018) Quantitative assessment of paravalvular leakage after transcatheter aortic valve replacement using a patient-specific pulsatile flow model. Int J Cardiol 258:313–320
Thubrikar MJ (1989) The aortic valve. CRC Press, Inc., Boca Raton, pp 157–174
Wu W et al (2015) Fluid–structure interaction model of a percutaneous aortic valve: comparison with an in vitro test and feasibility study in a patient-specific case. Ann Biomed Eng 44:590–603
Acknowledgements
Stony Brook University SeaWulf cluster provided the computational resources for this study. ANSYS, SIMULIA Living Heart Project, and Capvidia FlowVision are in academic partnerships with Prof. Danny Bluestein. We would like to thank Praveen Sridhar and Jiang Yao from Simulia and Sinan Soganci from Capvidia for their technical support throughout the project.
Funding
This study was supported by the NIH-NIBIB Quantum award Phase II-U01EB012487 (DB) and NIH-NIBIB 1U01EB026414-01 (DB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ghosh, R.P., Marom, G., Bianchi, M. et al. Numerical evaluation of transcatheter aortic valve performance during heart beating and its post-deployment fluid–structure interaction analysis. Biomech Model Mechanobiol 19, 1725–1740 (2020). https://doi.org/10.1007/s10237-020-01304-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-020-01304-9