Abstract
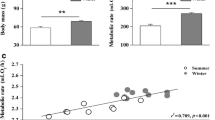
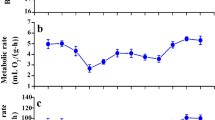
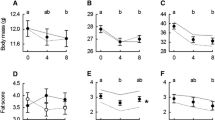
Phenotypic flexibility in avian metabolic rates and body composition have been well-studied in high-latitude species, which typically increase basal metabolic rate (BMR) and summit metabolism (Msum) when acclimatized to winter conditions. Patterns of seasonal metabolic acclimatization are more variable in lower-latitude birds that experience milder winters, with fewer studies investigating adjustments in avian organ and muscle masses in the context of metabolic flexibility in these regions. We quantified seasonal variation (summer vs winter) in the masses of organs and muscles frequently associated with changes in BMR (gizzard, intestines and liver) and Msum (heart and pectoral muscles), in white-browed sparrow-weavers (Plocepasser mahali). We also measured pectoral muscle thickness using a portable ultrasound system to determine whether we could non-lethally estimate muscle size. A concurrent study measured seasonal changes in BMR and Msum in the same population of sparrow-weavers, but different individuals. There was no seasonal variation in the dry masses of the gizzard, intestines or liver of sparrow-weavers, and during the same period, BMR did not vary seasonally. We found significantly higher heart (~ 18% higher) and pectoral muscle (~ 9% higher) dry mass during winter, although ultrasound measurements did not detect seasonal changes in pectoral muscle size. Despite winter increases in pectoral muscle mass, Msum was ~ 26% lower in winter compared to summer. To the best of our knowledge, this is the first study to report an increase in avian pectoral muscle mass but a concomitant decrease in thermogenic capacity.

Similar content being viewed by others
References
Barceló G, Love OP, Vézina F (2017) Uncoupling basal and summit metabolic rates in white-throated sparrows: digestive demand drives maintenance costs, but changes in muscle mass are not needed to improve thermogenic capacity. Physiol Biochem Zool 90:153–165
Bartoń K (2018) MuMIn: multi-model inference. R package version 1(42):1
Battley PF, Piersma T, Rogers DI, Dekinga A, Spaans B, Van Gils JA (2004) Do body condition and plumage during fuelling predict northwards departure dates of Great Knots Calidris tenuirostris from north-west Australia? Ibis 146:46–60
Cavieres G, Sabat P (2008) Geographic variation in the response to thermal acclimation in rufous-collared sparrows: are physiological flexibility and environmental heterogeneity correlated? Funct Ecol 22:509–515
Chappell MA, Bech C, Buttemer WA (1999) The relationship of central and peripheral organ masses to aerobic performance variation in house sparrows. J Exp Biol 202:2269–2279
Cooper SJ (2002) Seasonal metabolic acclimatization in mountain chickadees and Juniper titmice. Physiol Biochem Zool 75:386–395
Dietz MW, Dekinga A, Piersma T, Verhulst S (1999) Estimating organ size in small migrating shorebirds with ultrasonography: an intercalibration exercise. Physiol Biochem Zool 72:28–37
du Plessis MA (2005) White-browed sparrow-weaver (Plocepasser mahali). In: Hockey PAR, Dean WRJ, Ryan PG (eds) Roberts birds of Southern Africa. Trustees of the John Voelcker Bird Book Fund, Cape Town, pp 1006–1007
Ferguson JWH (1988) Dietary overlap in plocepasserine weavers (Aves: Ploceidae). S-Afr Tydskr Dierk 23:266–271
Hartman FA (1961) Locomotor mechanisms of birds. Smithson Misc Collect 143:1–91
Hohtola E (1982) Thermal and electromyographic correlates of shivering thermogenesis in the pigeon. Comp Biochem Physiol A 73:159–166
Hohtola E, Henderson RP, Rashotte ME (1998) Shivering thermogenesis in the pigeon: the effects of activity, diurnal factors, and feeding state. Am J Physiol Regul Integr Comp Physiol 275:R1553–R1562
Liknes ET, Swanson DL (2011) Phenotypic flexibility of body composition associated with seasonal acclimatization in passerine birds. J Therm Biol 36:363–370
Marsh RL, Dawson WR (1989) Avian adjustments to cold. In: Wang LCH (ed) Animal adaptation to cold. Springer, Berlin, pp 205–253
McKechnie AE, Noakes MJ, Smit BE (2015) Global patterns of seasonal acclimatization in avian resting metabolic rates. J Ornithol 156:367–376
McNab BK (1997) On the utility and uniformity in the definition of basal rate of metabolism. Physiol Zool 70:718–720
McWilliams SR, Karasov WH (2014) Spare capacity and phenotypic flexibility in the digestive system of a migratory bird: defining the limits of animal design. Proc R Soc B 281:20140308
Milbergue MS, Blier PU, Vézina F (2018) Large muscles are beneficial but not required for improving thermogenic capacity in small birds. Sci Rep 8:14009
Newton SF (1993) Body condition of a small passerine bird: ultrasonic assessment and significance in overwinter survival. J Zool Lond 229:561–580
Noakes MJ, McKechnie AE (2020) Seasonal metabolic acclimatization varies in direction and magnitude among years in two arid-zone passerines. Physiol Biochem Zool 93:140–152
Noakes MJ, Wolf BO, McKechnie AE (2017) Seasonal metabolic acclimatization varies in direction and magnitude among populations of an Afrotropical passerine bird. Physiol Biochem Zool 90:178–189
Petit M, Clavijo-Baquet S, Vézina F (2017) Increasing winter maximal metabolic rate improves intrawinter survival in small birds. Physiol Biochem Zool 90:166–177
Petit M, Lewden A, Vézina F (2013) Intra-seasonal flexibility in avian metabolic performance highlights the uncoupling of basal metabolic rate and thermogenic capacity. PLoS ONE 8:e68292
Petit M, Lewden A, Vézina F (2014) How does flexibility in body composition relate to seasonal changes in metabolic performance in a small passerine wintering at northern latitude? Physiol Biochem Zool 87(4):539–549
Petit M, Vézina F (2014a) Phenotype manipulations confirm the role of pectoral muscles and haematocrit in avian maximal thermogenic capacity. J Exp Biol 217:824–830
Petit M, Vézina F (2014b) Reaction norms in natural conditions: how does metabolic performance respond to weather variations in a small endotherm facing cold environments? PLoS ONE 9:e113617
Piersma T, Drent J (2003) Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol 18:228–233
Piersma T, van Gils JA (2010) The flexible phenotype: a body-centred integration of ecology, physiology, and behaviour. Oxford University Press, Oxford
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Royer-Boutin P, Cortés PA, Milbergue M, Petit M, Vézina F (2015) Estimation of muscle mass by ultrasonography differs between observers and life states of models in small birds. Physiol Biochem Zool 88:336–344
Sears J (1988) Assessment of body condition in live birds; measurements of protein and fat reserves in the mute swan, Cygnus olor. J Zool 216:295–308
Smit BE, McKechnie AE (2010) Avian seasonal metabolic variation in a subtropical desert: basal metabolic rates are lower in winter than in summer. Funct Ecol 24:330–339
Stager M, Swanson DL, Cheviron ZA (2015) Regulatory mechanisms of metabolic flexibility in the dark-eyed junco (Junco hyemalis). J Exp Biol 218:767–777
Swanson DL (1995) Seasonal variation in thermogenic capacity of migratory warbling vireos. Auk 112:870–877
Swanson DL (2001) Are summit metabolism and thermogenic endurance correlated in winter acclimatized passerine birds? J Comp Physiol B 171:475–481
Swanson DL (2010) Seasonal metabolic variation in birds: functional and mechanistic correlates. Curr Ornithol 17:75–129
Swanson DL, Dean KL (1995) Migration-induced variation in thermogenic capacity in migratory passerines. J Avian Biol 30:245–254
Swanson DL, King MO, Harmon E (2014a) Seasonal variation in pectoralis muscle and heart myostatin and tolloid-like proteinases in small birds: a regulatory role for seasonal phenotypic flexibility? J Comp Physiol B 184:249–258
Swanson DL, Liknes ET (2006) A comparative analysis of thermogenic capacity and cold tolerance in small birds. J Exp Biol 209:466–474
Swanson DL, Merkord C (2013) Seasonal phenotypic flexibility of flight muscle size in small birds: a comparison of ultrasonography and tissue mass measurements. J Ornithol 154:119–127
Swanson DL, Olmstead KL (1999) Evidence for a proximate influence of winter temperatures on metabolism in passerine birds. Physiol Biochem Zool 72:566–575
Swanson DL, Vézina F (2015) Environmental, ecological and mechanistic drivers of avian seasonal metabolic flexibility in response to cold winters. J Ornithol 156:S377–S388
Swanson DL, Zhang YF, King MO (2013) Individual variation in thermogenic capacity is correlated with flight muscle size but not cellular metabolic capacity in american goldfinches (Spinus tristis). Physiol Biochem Zool 86:421–431
Swanson DL, Zhang YF, Liu J-S, Merkord CL, King MO (2014b) Relative roles of temperature and photoperiod as drivers of metabolic flexibility in dark-eyed juncos. J Exp Biol 217:866–875
van de Ven TMFN, Mzilikazi N, McKechnie AE (2013) Seasonal metabolic variation in two populations of an Afrotropical euplectid bird. Physiol Biochem Zool 86:19–26
Vézina F, Dekinga A, Piersma T (2011) Shorebirds’ seasonal adjustments in thermogenic capacity are reflected by changes in body mass: how preprogrammed and instantaneous acclimation work together. Integr Comp Biol 51:394–408
Vézina F, Gerson AR, Guglielmo CG, Piersma T (2017) The performing animal: causes and consequences of body remodeling and metabolic adjustments in red knots facing contrasting thermal environments. Am J Physiol Regul Integr Comp Physiol 313:R120–R131
Vézina F, Jalvingh KM, Dekinga A, Piersma T (2007) Thermogenic side effects to migratory predisposition in shorebirds. Am J Physiol Regul Integr Comp Physiol 292:R1287–R1297
Vézina F, Williams TD (2005) Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European Starlings: implications for metabolic rate and organ mass relationships. Funct Ecol 19:119–128
Williams JB, Tieleman BI (2000) Flexibility in basal metabolic rate and evaporative water loss among hoopoe larks exposed to different environmental temperatures. J Exp Biol 203:3153–3159
Wolak ME, Fairbairn DJ, Paulsen YR (2012) Guidelines for estimating repeatability. Met Ecol Evol 3:129–137
Zhang Y, King MO, Harmon E, Swanson DL (2015) Summer-to-winter phenotypic flexibility of fatty acid transport and catabolism in skeletal muscle and heart of small birds. Physiol Biochem Zool 88:535–549
Zheng W-H, Li M, Liu J-S, Shao S-L, Xu X-J (2014a) Seasonal variation of metabolic thermogenesis in Eurasian tree sparrows (Passer montanus) over a latitudinal gradient. Physiol Biochem Zool 87:704–718
Zheng W-H, Liu J-S, Swanson DL (2014b) Seasonal phenotypic flexibility of body mass, organ masses, and tissue oxidative capacity and their relationship to resting metabolic rate in Chinese bulbuls. Physiol Biochem Zool 87:432–444
Acknowledgements
We thank the Rossouw family for allowing us to conduct research work on their property. We are grateful to Michelle Thompson, Sekgwari Malematja and Mervyn Uys for assistance in the field, and Janca Nortjé and Chuma Mateza for helping with laboratory work. We also thank Blair Wolf for the use of his portable ultrasound system. We obtained permission to conduct this research from the Animal Ethics Committee of the University of Pretoria (EC088-15) and the Department of Environment and Nature Conservation of the Northern Cape Province of South Africa (FAUNA 929/2/2015).
Funding
This work was supported by funding from the DST-NRF Centre of Excellence at the FitzPatrick Institute and the National Research Foundation of South Africa (Grant no. 110506 to AEM). Any opinions, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Research Foundation. WHK was supported by a Visiting Professorship from the University of Pretoria.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Noakes, M.J., Karasov, W.H. & McKechnie, A.E. Seasonal variation in body composition in an Afrotropical passerine bird: increases in pectoral muscle mass are, unexpectedly, associated with lower thermogenic capacity. J Comp Physiol B 190, 371–380 (2020). https://doi.org/10.1007/s00360-020-01273-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01273-6