Abstract
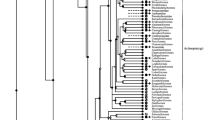
In most vertebrates, red blood cell carbonic anhydrase (RBC CA) plays a critical role in carbon dioxide (CO2) transport and excretion across epithelial tissues. Many early-diverging fishes (e.g., hagfish and chondrichthyans) are unique in possessing plasma-accessible membrane-bound CA-IV in the gills, allowing some CO2 excretion to occur without involvement from the RBCs. However, implications of this on RBC CA function are unclear. Through homology cloning techniques, we identified the putative protein sequences for RBC CA from nine early-diverging species. In all cases, these sequences contained a modification of the proton shuttle residue His-64, and activity measurements from three early-diverging fish demonstrated significantly reduced CA activity. Site-directed mutagenesis was used to restore the His-64 proton shuttle, which significantly increased RBC CA activity, clearly illustrating the functional significance of His-64 in fish red blood cell CA activity. Bayesian analyses of 55 vertebrate cytoplasmic CA isozymes suggested that independent evolutionary events led to the modification of His-64 and thus reduced CA activity in hagfish and chondrichthyans. Additionally, in early-diverging fish that possess branchial CA-IV, there is an absence of His-64 in RBC CAs and the absence of the Root effect [where a reduction in pH reduces hemoglobin’s capacity to bind with oxygen (O2)]. Taken together, these data indicate that low-activity RBC CA may be present in all fish with branchial CA-IV, and that the high-activity RBC CA seen in most teleosts may have evolved in conjunction with enhanced hemoglobin pH sensitivity.



Similar content being viewed by others
References
Alderman SL, Harter TS, Wilson JM, Supuran CT, Farrell AP, Brauner CJ (2016) Evidence for a plasma-accessible carbonic anhydrase in the lumen of salmon heart that may enhance oxygen delivery to the myocardium. J Exp Biol 219:719–724
Berenbrink M (2006) Evolution of vertebrate haemoglobins: histidine side chains, specific buffer value and Bohr effect. Resp Physiol Neurobiol 154:165–184
Berenbrink M, Bridges C (1994a) Active Na+–, Cl–and HCO3–dependent acid extrusion in Atlantic cod red blood cells in winter activated by hypercapnia. J Exp Biol 192:239–252
Berenbrink M, Bridges C (1994b) Catecholamine-activated sodium/proton exchange in the red blood cells of the marine teleost Gadus morhua. J Exp Biol 192:253–267
Berenbrink M, Koldkjær P, Kepp O, Cossins AR (2005) Evolution of oxygen secretion in fishes and the emergence of a complex physiological system. Science 307:1752–1757
Bohr C, Hasselbalch K, Krogh A (1904) Über einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt. Acta Physiol 16:402–412
Brauner C, Berenbrink M (2007) Gas transport and exchange. Fish physiology, vol 26. Academic Press, Cambridge, pp 213–282
Brauner C, Randall D (1998) The linkage between oxygen and carbon dioxide transport. Fish physiology, vol 17. Elsevier, Amsterdam, pp 283–319
Brittain T (2005) Root effect hemoglobins. J Inorg Biochem 99:120–129
Chegwidden WR, Carter ND (2000) Introduction to the carbonic anhydrases. The carbonic anhydrases. Birkhäuser, Basel, pp 13–28
Christianson DW, Fierke CA (1996) Carbonic anhydrase: evolution of the zinc binding site by nature and by design. Accounts Chem Res 29:331–339
Clifford AM, Weinrauch AM, Edwards SL, Wilkie MP, Goss GG (2017) Flexible ammonia handling strategies using both cutaneous and branchial epithelia in the highly ammonia-tolerant Pacific hagfish. Am J Physiol Reg I 313:R78–R90
Ellory J, Wolowyk M, Young J (1987) Hagfish (Eptatretus stouti) erythrocytes show minimal chloride transport activity. J Exp Biol 129:377–383
Esbaugh A, Tufts B (2006a) The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Resp Physiol Neurobiol 154:185–198
Esbaugh AJ, Tufts BL (2006b) Tribute to R. G. Boutilier: evidence of a high activity carbonic anhydrase isozyme in the red blood cells of an ancient vertebrate, the sea lamprey Petromyzon marinus. J Exp Biol 209:1169–1178
Esbaugh AJ, Lund SG, Tufts BL (2004) Comparative physiology and molecular analysis of carbonic anhydrase from the red blood cells of teleost fish. J Comp Physiol B 174:429–438
Esbaugh AJ, Gilmour K, Perry S (2009) Membrane-associated carbonic anhydrase in the respiratory system of the Pacific hagfish (Eptatretus stouti). Resp Physiol Neurobiol 166:107–116
Esbaugh AJ, Secor SM, Grosell M (2015) Characterization of carbonic anhydrase XIII in the erythrocytes of the Burmese python, Python molurus bivittatus. Comp Biochem Phys B 187:71–77
Ferguson R, Sehdev N, Bagatto B, Tufts B (1992) In vitro interactions between oxygen and carbon dioxide transport in the blood of the sea lamprey (Petromyzon marinus). J Exp Biol 173:25–41
Ferreira-Martins D, McCormick S, Campos A, Lopes-Marques M, Osório H, Castro LFC, Wilson JM (2016) A cytosolic carbonic anhydrase molecular switch occurs in the gills of metamorphic sea lamprey. Sci Rep 6:339–354
Forster R, Steen J (1968) Rate limiting processes in the Bohr shift in human red cells. J Physiol 196:541–562
Geers C, Gros G (2000) Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 80:681–715
Giardina B, Mosca D, De Rosa M (2004) The Bohr effect of haemoglobin in vertebrates: an example of molecular adaptation to different physiological requirements. Acta Physiol 182:229–244
Gilmour K (2010) Perspectives on carbonic anhydrase. Comp Biochem Phys A 157:193–197
Gilmour K, Perry S (2009) Carbonic anhydrase and acid–base regulation in fish. J Exp Biol 212:1647–1661
Gilmour KM, Henry RP, Wood CM, Perry SF (1997) Extracellular carbonic anhydrase and an acid-base disequilibrium in the blood of the dogfish, Squalus acanthias. J Exp Biol 200:173–183
Gilmour K, Perry S, Bernier N, Henry R, Wood C (2001) Extracellular carbonic anhydrase in the dogfish, Squalus acanthias: a role in CO2 excretion. Physiol Biochem Zool 74:477–492
Gilmour K, Bayaa M, Kenney L, Mcneill B, Perry S (2007) Type IV carbonic anhydrase is present in the gills of spiny dogfish (Squalus acanthias). Am J Physiol-Reg I 292:R556–R567
Gilmour KM, Perry SF (2004) Branchial membrane-associated carbonic anhydrase activity maintains CO2 excretion in severely anemic dogfish. Am J Physiol-Reg I 286:R1138–R1148
Gilmour KM, Shah B, Szebedinszky C (2002) An investigation of carbonic anhydrase activity in the gills and blood plasma of brown bullhead (Ameiurus nebulosus), longnose skate (Raja rhina), and spotted raffish (Hydrolagus colliei). J Comp Physiol B 172:77–86
Harter TS, Brauner CJ (2017) The O2 and CO2 transport system in teleosts and the specialized mechanisms that enhance Hb–O2 unloading to tissues. Fish physiolpgy, vol 36. Academic Press, Cambridge, pp 1–106
Harter TS, Zanuzzo FS, Supuran CT, Gamperl AK, Brauner CJ (2019) Functional support for a novel mechanism that enhances tissue oxygen extraction in a teleost fish. In: Proceedings of the Royal Society B, p 286
Henry RP (1991) Techniques for measuring carbonic anhydrase activity in vitro: the electrometric delta pH and pH statmethods. The carbonic anhydrases. Springer, New York, pp 119–125
Henry RP, Swenson ER (2000) The distribution and physiological significance of carbonic anhydrase in vertebrate gas exchange organs. Resp Physiol 121:1–12
Henry RP, Tufts BL, Boutilier RG (1993) The distribution of carbonic anhydrase type I and II isozymes in lamprey and trout: possible co-evolution with erythrocyte chloride/bicarbonate exchange. J Comp Physiol B 163:380–388
Henry RP, Gilmour KM, Wood CM, Perry SF (1997) Extracellular carbonic anhydrase activity and carbonic anhydrase inhibitors in the circulatory system of fish. Physiol Zool 70:650–659
Jensen FB (1999) Haemoglobin H+ equilibria in lamprey (Lampetra fluviatilis) and hagfish (Myxine glutinosa). J Exp Biol 202:1963–1968
Jensen FB (2004) Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiologica 182:215–227
Lindskog S, Silverman DN (2000) The catalytic mechanism of mammalian carbonic anhydrases. The carbonic anhydrases. Birkhäuser, Basel, pp 175–195
Maren TH, Swenson ER (1980) A comparative study of the kinetics of the Bohr effect in vertebrates. J Physiol 303:535–547
Maren TH, Friedlandl BR, Rittmaster RS (1980) Kinetic properties of primitive vertebrate carbonic anhydrases. Comp Biochem Physiol B 67:69–74
McMillan OJL, Dichiera AM, Harter TS, Wilson JM, Esbaugh AJ, Brauner CJ (2019) Blood and gill carbonic anhydrase in the context of a chondrichthyan model of CO2 excretion. Phys Biochem Zool 92(6):554–566
Morrison PR, Gilmour KM, Brauner CJ (2015) Oxygen and carbon dioxide transport in elasmobranchs. Fish physiology, vol 34. Academic Press, Cambridge, pp 127–219
Nikinmaa M (1986) Red cell pH of lamprey (Lampetra fluviatilis) is actively regulated. J Comp Physiol B 156:747–750
Nikinmaa M (1993) Haemoglobin function in intact Lampetra fluviatilis erythrocytes. Resp Physiol 91:283–293
Nikinmaa M, Huestis WH (1984) Adrenergic swelling of nucleated erythrocytes: cellular mechanisms in a bird, domestic goose, and two teleosts, striped bass and rainbow trout. J Exp Biol 113:215–224
Nikinmaa M, Airaksinen S, Virkki LV (1995) Haemoglobin function in intact lamprey erythrocytes: interactions with membrane function in the regulation of gas transport and acid-base balance. J Exp Biol 198:2423–2430
Nikinmaa M, Berenbrink M, Brauner CJ (2019) Regulation of erythrocyte function: multiple evolutionary solutions for respiratory gas transport and its regulation in fish. Acta Physiol 227(2):e13299
Pelster B, Weber R (1991) The Physiology of the Root Effect. Advances in comparative and environmental physiology. Springer, New York, pp 51–77
Randall D, Rummer J, Wilson J, Wang S, Brauner C (2014) A unique mode of tissue oxygenation and the adaptive radiation of teleost fishes. J Exp Biol 217:1205–1214
Ronquist F, Teslenko M, Van Der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Root R (1931) The respiratory function of the blood of marine fishes. Biol Bull 61:427–456
Rummer JL, Brauner CJ (2011) Plasma-accessible carbonic anhydrase at the tissue of a teleost fish may greatly enhance oxygen delivery: in vitro evidence in rainbow trout, Oncorhynchus mykiss. J Exp Biol 214:2319–2328
Rummer JL, Brauner CJ (2015) Root effect haemoglobins in fish may greatly enhance general oxygen delivery relative to other vertebrates. PloS One 10:e0139477
Rummer JL, Mckenzie DJ, Innocenti A, Supuran CT, Brauner CJ (2013) Root effect hemoglobin may have evolved to enhance general tissue oxygen delivery. Science 340:1327–1329
Scholander PF, Van Dam L (1954) Secretion of gases against high pressures in the swimbladder of deep sea fishes, I. Oxygen dissociation in blood. Biol Bull 107:247–259
Silverman DN, Lindskog S (1988) The catalytic mechanism of carbonic anhydrase: implications of a rate-limiting protolysis of water. Accounts Chem Res 21:30–36
Stams T, Christianson DW (2000) X-ray crystallographic studies of mammalian carbonic anhydrase isozymes. The carbonic anhydrases. Birkhäuser, Basel, pp 159–174
Swenson ER (2000) Respiratory and renal roles of carbonic anhydrase in gas exchange and acid-base regulation. The carbonic anhydrases. Birkhäuser, Basel, pp 281–341
Takezaki N, Figueroa F, Zaleska-Rutczynska Z, Klein J (2003) Molecular phylogeny of early vertebrates: monophyly of the agnathans as revealed by sequences of 35 genes. Mol Biol Evol 20:287–292
Tashian RE (1992) Genetics of the mammalian carbonic anhydrases. Advances in genetics, vol 30. Academic Press, Cambridge, pp 321–356
Tu C, Silverman DN, Forsman C, Jonsson BH, Lindskog S (1989) Role of histidine 64 in the catalytic mechanism of human carbonic anhydrase II studied with a site-specific mutant. Biochem 28:7913–7918
Tufts B, Perry SF (1998) Carbon dioxide transport and excretion. Fish physiology, vol 17. Academic Press, Cambridge, pp 229–281
Wang Q, Arighi CN, King BL, Polson SW, Vincent J, Chen C, Huang H, Kingham BF, Page ST, Farnum RM, Thomas WK, Udwary DW, Wu CH, The North East Bioinformatics Collaborative Curation, T (2012) Community annotation and bioinformatics workforce development in concert—Little Skate Genome Annotation Workshops and Jamborees. Database, 2012
Wells R, Forster M, Davison W, Taylor H, Davie P, Satchell G (1986) Blood oxygen transport in the free-swimming hagfish, Eptatretus cirrhatus. J Exp Biol 123:43–53
Wilson JM, Randall DJ, Vogl AW, Harris J, Sly WS, Iwama GK (2000) Branchial carbonic anhydrase is present in the dogfish Squalus acanthias. Fish Physiol Biochem 22:329–336
Wyffels J, King BL, Vincent J, Chen C, Wu CH, Polson SW (2014) SkateBase, an elasmobranch genome project and collection of molecular resources for chondrichthyan fishes. F1000es 3:191
Acknowledgements
This study was funded by the National Science Foundation Grant to A.J.E. (EF 1315290). The authors would like to thank everyone who kindly donated or helped obtain blood samples: Dr. Jim Gelsleichter, The University of North Florida; Dr. Till Harter, Ellen Jung, Dr. Phill Morrison, and Dr. Chris M. Wood, University of British Columbia; Dr. C. Sepulveda, Pflüger Institute for Environmental Research; Dr. Diego Bernal, UMass Amherst; Dr. Martin Haulena and the veterinary team, Vancouver Aquarium; Dr. Adalberto Val, National Institute for Research of the Amazon; Dr. Gary Anderson, University of Manitoba; the research and foreshore staff at Bamfield Marine Sciences Centre, Bamfield, BC. Additionally, the authors gratefully acknowledge Dr. Kiley Seitz and Dr. Nina Dombrowski for their expertise and guidance in Bayesian phylogenetics. No competing interests declared.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.V. Carey.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dichiera, A.M., McMillan, O.J.L., Clifford, A.M. et al. The importance of a single amino acid substitution in reduced red blood cell carbonic anhydrase function of early-diverging fish. J Comp Physiol B 190, 287–296 (2020). https://doi.org/10.1007/s00360-020-01270-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01270-9