Abstract
Purpose
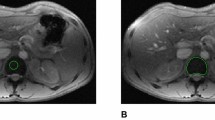
A prospective study was conducted to evaluate signal changes in the dentate nucleus, globus pallidus, pons, and thalamus (normalized to the deep cerebellum white matter) in T1-weighted magnetic resonance (MR) images after serial injections of gadobutrol in patients with thalassemia without neurological lesions.
Methods
In this study three groups were scanned at both 1.5 T and 3 T: 15 thalassemia patients transfused and chelated with ≥4 gadobutrol administrations at a high dose (0.2 mmol/kg per scan) for late gadolinium enhancement (LGE) cardiovascular MR, 8 thalassemia patients and 13 healthy subjects who had never received gadolinium-based contrast agents (GBCA).
Results
Signal intensity (SI) ratios at 1.5 T in all regions were comparable among the three groups and were not correlated with the number of gadobutrol administrations.
In healthy subjects SI ratios were significantly different among the 4 regions, being higher in the pallidus.
The SI ratios at 1.5 T were significantly higher and not correlated with SI ratios at 3 T or with iron overload in the same regions assessed by the T2* technique.
Conclusion
This article describes the lack of increased SI in T1-weighted MR images after repeated administration of gadobutrol for cardiovascular MR studies in a high-risk population (high dose per scan, iron overload that can facilitate the transmetalation of gadolinium) scanned at 3 T and 1.5 T.



Similar content being viewed by others
References
Claussen C, Laniado M, Kazner E, Schörner W, Felix R. Application of contrast agents in CT and MRI (NMR): their potential in imaging of brain tumors. Neuroradiology. 1985;27:164–71.
Niendorf HP, Felix R, Laniado M, Schörner W, Claussen C, Weinmann HJ. Gadolinium-DTPA: a new contrast agent for magnetic resonance imaging. Radiat Med. 1985;3:7–12.
Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94:3318–26.
Bruder O, Wagner A, Lombardi M, Schwitter J, van Rossum A, Pilz G, et al. European Cardiovascular Magnetic Resonance (EuroCMR) registry—multi national results from 57 centers in 15 countries. J Cardiovasc Magn Reson. 2013;15:9.
Grobner T. Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–8.
Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359–62.
Frenzel T, Lengsfeld P, Schirmer H, Hutter J, Weinmann HJ. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008;43:817–28.
Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270:834–41.
Errante Y, Cirimele V, Mallio CA, Di Lazzaro V, Zobel BB, Quattrocchi CC. Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Invest Radiol. 2014;49:685–90.
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276:228–32.
Quattrocchi CC, Mallio CA, Errante Y, Cirimele V, Carideo L, Ax A, et al. Gadodiamide and dentate nucleus T1 hyperintensity in patients with meningioma evaluated by multiple follow-up contrast-enhanced magnetic resonance examinations with no systemic interval therapy. Invest Radiol. 2015;50:470–2.
Radbruch A, Weberling LD, Kieslich PJ, Eidel O, Burth S, Kickingereder P, et al. Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology. 2015;275:783–91.
Ramalho J, Castillo M, AlObaidy M, Nunes RH, Ramalho M, Dale BM, et al. High signal intensity in globus pallidus and dentate nucleus on Unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology. 2015;276:836–44.
Weberling LD, Kieslich PJ, Kickingereder P, Wick W, Bendszus M, Schlemmer HP, et al. Increased signal intensity in the dentate nucleus on Unenhanced T1-weighted images after gadobenate dimeglumine administration. Invest Radiol. 2015;50:743–8.
Adin ME, Kleinberg L, Vaidya D, Zan E, Mirbagheri S, Yousem DM. Hyperintense dentate nuclei on T1-weighted MRI: relation to repeat gadolinium administration. AJNR Am J Neuroradiol. 2015;36:1859–65.
Cao Y, Huang DQ, Shih G, Prince MR. Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol. 2016;206:414–9.
Ramalho J, Semelka RC, AlObaidy M, Ramalho M, Nunes RH, Castillo M. Signal intensity change on unenhanced T1-weighted images in dentate nucleus following gadobenate dimeglumine in patients with and without previous multiple administrations of gadodiamide. Eur Radiol. 2016;26:4080–8.
Schlemm L, Chien C, Bellmann-Strobl J, Dörr J, Wuerfel J, Brandt AU, et al. Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler. 2017;23:963–72.
Tanaka M, Nakahara K, Kinoshita M. Increased signal intensity in the dentate nucleus of patients with multiple sclerosis in comparison with neuromyelitis optica spectrum disorder after multiple doses of gadolinium contrast. Eur Neurol. 2016;75:195–8.
Zhang Y, Cao Y, Shih GL, Hecht EM, Prince MR. Extent of signal hyperintensity on Unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology. 2016;282:516–25.
Radbruch A, Weberling LD, Kieslich PJ, Hepp J, Kickingereder P, Wick W, et al. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Invest Radiol. 2015;50:805–10.
Eisele P, Alonso A, Szabo K, Ebert A, Ong M, Schoenberg SO, et al. Lack of increased signal intensity in the dentate nucleus after repeated administration of a macrocyclic contrast agent in multiple sclerosis: An observational study. Medicine. 2016;95:e4624.
Yoo RE, Sohn CH, Kang KM, Yun TJ, Choi SH, Kim JH, et al. Evaluation of gadolinium retention after serial administrations of a macrocyclic gadolinium-based contrast agent (Gadobutrol): a single-institution experience with 189 patients. Invest Radiol. 2018;53:20–5.
Müller A, Jurcoane A, Mädler B, Ditter P, Schild H, Hattingen E. Brain relaxometry after macrocyclic Gd-based contrast agent. Clin Neuroradiol. 2017;27:459–68.
McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275:772–82.
Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51:447–53.
Ramalho J, Ramalho M, AlObaidy M, Semelka RC. Technical aspects of MRI signal change quantification after gadolinium-based contrast agents’ administration. Magn Reson Imaging. 2016;34:1355–8.
Meloni A, Ramazzotti A, Positano V, Salvatori C, Mangione M, Marcheschi P, et al. Evaluation of a web-based network for reproducible T2* MRI assessment of iron overload in thalassemia. Int J Med Inform. 2009;78:503–12.
Ramazzotti A, Pepe A, Positano V, Rossi G, De Marchi D, Brizi MG, et al. Multicenter validation of the magnetic resonance t2* technique for segmental and global quantification of myocardial iron. J Magn Reson Imaging. 2009;30:62–8.
Meloni A, Favilli B, Positano V, Cianciulli P, Filosa A, Quarta A, et al. Safety of cardiovascular magnetic resonance gadolinium chelates contrast agents in patients with hemoglobinopaties. Haematologica. 2009;94:1625–7.
Pepe A, Positano V, Capra M, Maggio A, Pinto CL, Spasiano A, et al. Myocardial scarring by delayed enhancement cardiovascular magnetic resonance in thalassaemia major. Heart. 2009;95:1688–93.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70.
Quattrocchi CC, Ramalho J, van der Molen AJ, Rovira À, Radbruch A; GREC, European Gadolinium Retention Evaluation Consortium and the ESNR, European Society of Neuroradiology. Standardized assessment of the signal intensity increase on unenhanced T1-weighted images in the brain: the European Gadolinium Retention Evaluation Consortium (GREC) Task Force position statement. Eur Radiol. 2019;29:3959–67.
Positano V, Pepe A, Santarelli MF, Scattini B, De Marchi D, Ramazzotti A, et al. Standardized T2* map of normal human heart in vivo to correct T2* segmental artefacts. NMR Biomed. 2007;20:578–90.
Stojanov DA, Aracki-Trenkic A, Vojinovic S, Benedeto-Stojanov D, Ljubisavljevic S. Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol. 2016;26:807–15.
Kromrey ML, Liedtke KR, Ittermann T, Langner S, Kirsch M, Weitschies W, et al. Intravenous injection of gadobutrol in an epidemiological study group did not lead to a difference in relative signal intensities of certain brain structures after 5 years. Eur Radiol. 2017;27:772–7.
Kim RJ, Wu E, Rafael A, Chen EL, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445–53.
Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002.
Tweedle MF, Hagan JJ, Kumar K, Mantha S, Chang CA. Reaction of gadolinium chelates with endogenously available ions. Magn Reson Imaging. 1991;9:409–15.
Akhlaghpoor S, Ghahari A, Morteza A, Khalilzadeh O, Shakourirad A, Alinaghizadeh MR. Quantitative T2* magnetic resonance imaging for evaluation of iron deposition in the brain of beta-thalassemia patients. Clin Neuroradiol. 2012;22:211–7.
Metafratzi Z, Argyropoulou MI, Kiortsis DN, Tsampoulas C, Chaliassos N, Efremidis SC. T(2) relaxation rate of basal ganglia and cortex in patients with beta-thalassaemia major. Br J Radiol. 2001;74:407–10.
Douthat WG, Acuña Aguerre G, Fernández Martín JL, Mouzo R, Cannata Andía JB. Treatment of aluminium intoxication: a new scheme for desferrioxamine administration. Nephrol Dial Transplant. 1994;9:1431–4.
Amiri A, Fatemi SJ, Fatemi SN. Removal of thallium by combining desferrioxamine and deferiprone chelators in rats. Biometals. 2007;20:159–63.
Kontoghiorghes GJ. New concepts of iron and aluminium chelation therapy with oral L1 (deferiprone) and other chelators. A review. Analyst. 1995;120:845–51.
Fatemi SJ, Khajoee Nejad F, Zandevakili T, Dahoee Balooch F. Chelation of cobalt by combining deferasirox, deferiprone and desferrioxamine in rats. Toxin Rev. 2013;33:146–50.
Maximova N, Gregori M, Zennaro F, Sonzogni A, Simeone R, Zanon D. Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology. 2016;281:418–26.
Lancelot E, Raynaud JS, Ferrari N, Desche P. Hepatic gadolinium deposition in pediatric hematopoietic stem cell transplant recipients. Radiology. 2016;281:982–3.
Acknowledgements
We would like to thank all the radiologists and thalassemologists of the MIOT and eMIOT projects. A thanks goes to all the radiology technicians and nurses in our Lab for their cooperation, helpfulness and patience. We finally thank all patients and healthy subjects who underwent MRI for their double cooperation and Claudia Santarlasci for skillful secretarial work.
Funding
The MIOT and EMIOT projects receive no-profit support from industrial sponsorships (Chiesi Farmaceutici S.p.A., ApoPharma Inc., and Bayer).
Author information
Authors and Affiliations
Contributions
Conduction of the study, statistical analysis, data interpretation, and manuscript drafting: Antonella Meloni; method development, image analysis, data interpretation, and manuscript revision: Domenico Montanaro; MRI scanning and manuscript revision: Daniele De Marchi and Petra Keilberg; method development, data interpretation, and manuscript revision: Mariachiara Resta and Sara De Cori; clinical data collection, volunteer recruitment, and manuscript revision: Laura Pistoia; software development and manuscript revision: Vincenzo positano; patient recruitment and manuscript revision: Anna Spasiano, Tommaso Casini, and Caterina Cinzia De Bari; study conception, design and coordination, data interpretation, and manuscript drafting: Alessia Pepe. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A. Meloni,D. Montanaro, D. De Marchi, M. Resta, P. Keilberg, L. Pistoia, V. Positano,A. Spasiano, T. Casini, C.C. De Bari, S. De Cori and A. Pepe declare that they have no competing interests.
Ethical standards
This prospective study was approved by the local ethics committee and was conducted in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave informed consent prior to inclusion in the study.
Rights and permissions
About this article
Cite this article
Meloni, A., Montanaro, D., De Marchi, D. et al. Absence of T1 Hyperintensity in the Brain of High-risk Patients After Multiple Administrations of High-dose Gadobutrol for Cardiac Magnetic Resonance. Clin Neuroradiol 31, 347–355 (2021). https://doi.org/10.1007/s00062-020-00897-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-020-00897-z