Abstract
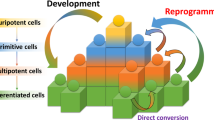
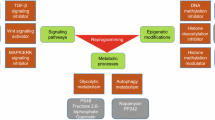
The ground state of embryonic stem cells (ESCs) is closely related to the development of regenerative medicine. Particularly, long-term culture of ESCs in vitro, maintenance of their undifferentiated state, self-renewal and multi-directional differentiation ability is the premise of ESCs mechanism and application research. Induced pluripotent stem cells (iPSC) reprogrammed from mouse embryonic fibroblasts (MEF) cells into cells with most of the ESC characteristics show promise towards solving ethical problems currently facing stem cell research. However, integration into chromosomal DNA through viral-mediated genes may activate proto oncogenes and lead to risk of cancer of iPSC. At the same time, iPS induction efficiency needs to be further improved to reduce the use of transcription factors. In this review, we discuss small molecules that promote self-renewal and reprogramming, including growth factor receptor inhibitors, GSK-3β and histone deacetylase inhibitors, metabolic regulators, pathway modulators as well as EMT/MET regulation inhibitors to enhance maintenance of ESCs and enable reprogramming. Additionally, we summarize the mechanism of action of small molecules on ESC self-renewal and iPSC reprogramming. Finally, we will report on the progress in identification of novel and potentially effective agents as well as selected strategies that show promise in regenerative medicine. On this basis, development of more small molecule combinations and efficient induction of chemically induced pluripotent stem cell (CiPSC) is vital for stem cell therapy. This will significantly improve research in pathogenesis, individualized drug screening, stem cell transplantation, tissue engineering and many other aspects.


Similar content being viewed by others
Abbreviations
- ESCs:
-
Embryonic stem cells
- iPSC:
-
Induced pluripotent stem cells
- GSK-3β:
-
Glycogen synthesis kinase 3β
- BIO:
-
6-bromoindirubin-3′-oxime
- MAPK:
-
Mitogen Activated Protein Kinase
- ERK:
-
Extracellular Regulated protein Kinase
- RasGAP:
-
RasGTP-activated protein
- PI3K:
-
Phosphatidyl inositol-3-kinase
- PDK1:
-
Phosphoinositide-dependent kinase-1
- MET:
-
Mesenchymal cells to epithelial cells
References
Martin, G. R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences of the United States of America, 78(12), 7634–7638.
Evans, M. J., & Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature, 292(5819), 154–156.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
Mikkelsen, T.S.et al., Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature, 2007. 448(7153): p. 553–560.
Okita, K., Ichisaka, T., & Yamanaka, S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature, 448(7151), 313–317.
Wernig, M., et al. (2007). In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature, 448(7151), 318–3U2.
Boland, M. J., Hazen, J. L., Nazor, K. L., Rodriguez, A. R., Gifford, W., Martin, G., Kupriyanov, S., & Baldwin, K. K. (2009). Adult mice generated from induced pluripotent stem cells. Nature, 461(7260), 91–U94.
Zhao, X. Y., et al. (2009). iPS cells produce viable mice through tetraploid complementation. Nature, 461(7260), 86–U88.
Kang, L., Wang, J., Zhang, Y., Kou, Z., & Gao, S. (2009). iPS cells can support full-term development of Tetraploid blastocyst-complemented embryos. Cell Stem Cell, 5(2), 135–138.
Park, I. H., Lerou, P. H., Zhao, R., Huo, H., & Daley, G. Q. (2008). Generation of human-induced pluripotent stem cells. Nature Protocols, 3(7), 1180–1186.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872.
Yu, J. Y., et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318(5858), 1917–1920.
Esteban, M. A., Xu, J., Yang, J., Peng, M., Qin, D., Li, W., Jiang, Z., Chen, J., Deng, K., Zhong, M., Cai, J., Lai, L., & Pei, D. (2009). Generation of induced pluripotent stem cell lines from Tibetan miniature pig. The Journal of Biological Chemistry, 284(26), 17634–17640.
Li, W., Wei, W., Zhu, S., Zhu, J., Shi, Y., Lin, T., Hao, E., Hayek, A., Deng, H., & Ding, S. (2009). Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell, 4(1), 16–19.
Liao, J., et al. (2009). Generation of induced pluripotent stem cell lines from adult rat cells. Cell Stem Cell, 4(1), 11–15.
Liu, H., Zhu, F., Yong, J., Zhang, P., Hou, P., Li, H., Jiang, W., Cai, J., Liu, M., Cui, K., Qu, X., Xiang, T., Lu, D., Chi, X., Gao, G., Ji, W., Ding, M., & Deng, H. (2008). Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell, 3(6), 587–590.
Koh, S., & Piedrahita, J. A. (2015). Generation of induced pluripotent stem cells (iPSCs) from adult canine fibroblasts. Methods in Molecular Biology, 1330, 69–78.
Wu, Z., Chen, J., Ren, J., Bao, L., Liao, J., Cui, C., Rao, L., Li, H., Gu, Y., Dai, H., Zhu, H., Teng, X., Cheng, L., & Xiao, L. (2009). Generation of pig induced pluripotent stem cells with a drug-inducible system. Journal of Molecular Cell Biology, 1(1), 46–54.
Roberts, R. M., Telugu, B. P., & Ezashi, T. (2009). Induced pluripotent stem cells from swine (sus scrofa): Why they may prove to be important. Cell Cycle, 8(19), 3078–3081.
Dimos, J. T., Rodolfa, K. T., Niakan, K. K., Weisenthal, L. M., Mitsumoto, H., Chung, W., Croft, G. F., Saphier, G., Leibel, R., Goland, R., Wichterle, H., Henderson, C. E., & Eggan, K. (2008). Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science, 321(5893), 1218–1221.
Ebert, A. D., Yu, J., Rose FF Jr, Mattis, V. B., Lorson, C. L., Thomson, J. A., & Svendsen, C. N. (2009). Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature, 457(7227), 277–2U1.
Soldner, F., Hockemeyer, D., Beard, C., Gao, Q., Bell, G. W., Cook, E. G., Hargus, G., Blak, A., Cooper, O., Mitalipova, M., Isacson, O., & Jaenisch, R. (2009). Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell, 136(5), 964–977.
Li, H. D., & Chen, G. (2016). In vivo reprogramming for CNS repair: Regenerating neurons from endogenous glial cells. Neuron, 91(4), 728–738.
Hanna, J., Wernig, M., Markoulaki, S., Sun, C. W., Meissner, A., Cassady, J. P., Beard, C., Brambrink, T., Wu, L. C., Townes, T. M., & Jaenisch, R. (2007). Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science, 318(5858), 1920–1923.
Kim, J. B., et al. (2009). Oct4-induced Pluripotency in adult neural stem cells. Cell, 136(3), 411–419.
Stadtfeld, M., Nagaya, M., Utikal, J., Weir, G., & Hochedlinger, K. (2008). Induced pluripotent stem cells generated without viral integration. Science, 322(5903), 945–949.
Aoi, T., Yae, K., Nakagawa, M., Ichisaka, T., Okita, K., Takahashi, K., Chiba, T., & Yamanaka, S. (2008). Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science, 321(5889), 699–702.
Stadtfeld, M., Brennand, K., & Hochedlinger, K. (2008). Reprogramming of pancreatic beta cells into induced pluripotent stem cells. Current Biology, 18(12), 890–894.
Hanna, J., Markoulaki, S., Schorderet, P., Carey, B. W., Beard, C., Wernig, M., Creyghton, M. P., Steine, E. J., Cassady, J. P., Foreman, R., Lengner, C. J., Dausman, J. A., & Jaenisch, R. (2008). Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell, 133(2), 250–264.
Broccoli, V. (2017). Reprogramming of somatic cells: iPS and iN cells. Progress in Brain Research, 230, 53–68.
Li, Y. Q., et al. (2011). Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Research, 21(1), 196–204.
Greder, L. V., Post, J., & Dutton, J. R. (2016). Using Oct4:MerCreMer lineage tracing to monitor endogenous Oct4 expression during the reprogramming of fibroblasts into induced pluripotent stem cells (iPSCs). Methods in Molecular Biology, 1357, 97–110.
Hou, P. P., et al. (2013). Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science, 341(6146), 651–654.
Zhao, T., et al. (2018). Single-cell RNA-Seq reveals dynamic early embryonic-like programs during chemical reprogramming. Cell Stem Cell,23(1), 31.
Ying, Q. L., et al. (2003). BMP induction of id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell, 115(3), 281–292.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S., & Jones, J. M. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Daheron, L., et al. (2004). LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells, 22(5), 770–778.
Ginis, I., Luo, Y., Miura, T., Thies, S., Brandenberger, R., Gerecht-Nir, S., Amit, M., Hoke, A., Carpenter, M. K., Itskovitz-Eldor, J., & Rao, M. S. (2004). Differences between human and mouse embryonic stem cells. Developmental Biology, 269(2), 360–380.
Sato, N., Meijer, L., Skaltsounis, L., Greengard, P., & Brivanlou, A. H. (2004). Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nature Medicine, 10(1), 55–63.
Richards, M., Tan, S. P., Tan, J. H., Chan, W. K., & Bongso, A. (2004). The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells, 22(1), 51–64.
Ying, Q. L., Wray, J., Nichols, J., Batlle-Morera, L., Doble, B., Woodgett, J., Cohen, P., & Smith, A. (2008). The ground state of embryonic stem cell self-renewal. Nature, 453(7194), 519–523.
Schugar, R. C., Robbins, P. D., & Deasy, B. M. (2008). Small molecules in stem cell self-renewal and differentiation. Gene Therapy, 15(2), 126–135.
Miyabayashi, T., Teo, J. L., Yamamoto, M., McMillan, M., Nguyen, C., & Kahn, M. (2007). Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proceedings of the National Academy of Sciences of the United States of America, 104(13), 5668–5673.
Chen, S., Do, J. T., Zhang, Q., Yao, S., Yan, F., Peters, E. C., Schöler, H. R., Schultz, P. G., & Ding, S. (2006). Self-renewal of embryonic stem cells by a small molecule. Proceedings of the National Academy of Sciences of the United States of America, 103(46), 17266–17271.
Heo, J. S., Lee, Y. J., & Han, H. J. (2006). EGF stimulates proliferation of mouse embryonic stem cells: Involvement of Ca2+ influx and p44/42 MAPKs. American Journal of Physiology. Cell Physiology, 290(1), C123–C133.
Watanabe, K., Ueno, M., Kamiya, D., Nishiyama, A., Matsumura, M., Wataya, T., Takahashi, J. B., Nishikawa, S., Nishikawa, S., Muguruma, K., & Sasai, Y. (2007). A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nature Biotechnology, 25(6), 681–686.
Dutta, D., Ray, S., Home, P., Larson, M., Wolfe, M. W., & Paul, S. (2011). Self-renewal versus lineage commitment of embryonic stem cells: Protein kinase C signaling shifts the balance. Stem Cells, 29(4), 618–628.
Chen, G., et al. (2014). Blocking autocrine VEGF signaling by sunitinib, an anti-cancer drug, promotes embryonic stem cell self-renewal and somatic cell reprogramming. Cell Research, 24(9), 1121–1136.
Aberle, H., Bauer, A., Stappert, J., Kispert, A., & Kemler, R. (1997). Beta-catenin is a target for the ubiquitin-proteasome pathway. The EMBO Journal, 16(13), 3797–3804.
Murray, J. T., Campbell, D. G., Morrice, N., Auld, G. C., Shpiro, N., Marquez, R., Peggie, M., Bain, J., Bloomberg, G. B., Grahammer, F., Lang, F., Wulff, P., Kuhl, D., & Cohen, P. (2004). Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. The Biochemical Journal, 384, 477–488.
Li, W., Zhou, H., Abujarour, R., Zhu, S., Young Joo, J., Lin, T., Hao, E., Schöler, H. R., Hayek, A., & Ding, S. (2009). Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells, 27(12), 2992–3000.
Johnson, G. L., & Lapadat, R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science, 298(5600), 1911–1912.
Armstrong, L., Hughes, O., Yung, S., Hyslop, L., Stewart, R., Wappler, I., Peters, H., Walter, T., Stojkovic, P., Evans, J., Stojkovic, M., & Lako, M. (2006). The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Human Molecular Genetics, 15(11), 1894–1913.
Kolanus, W., & Seed, B. (1997). Integrins and inside-out signal transduction: Converging signals from PKC and PIP3. Current Opinion in Cell Biology, 9(5), 725–731.
Huangfu, D. W., et al. (2008). Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nature Biotechnology, 26(7), 795–797.
Kang, S. J., Park, Y. I., So, B., & Kang, H. G. (2014). Sodium butyrate efficiently converts fully reprogrammed induced pluripotent stem cells from mouse partially reprogrammed cells. Cell Reports, 16(5), 345–354.
Shi, Y., Desponts, C., Do, J. T., Hahm, H. S., Schöler, H. R., & Ding, S. (2008). Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell, 3(5), 568–574.
Shi, Y., et al. (2008). A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell Stem Cell, 2(6), 525–528.
Wang, Q., Xu, X., Li, J., Liu, J., Gu, H., Zhang, R., Chen, J., Kuang, Y., Fei, J., Jiang, C., Wang, P., Pei, D., Ding, S., & Xie, X. (2011). Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells. Cell Research, 21(10), 1424–1435.
Esteban, M. A., Wang, T., Qin, B., Yang, J., Qin, D., Cai, J., Li, W., Weng, Z., Chen, J., Ni, S., Chen, K., Li, Y., Liu, X., Xu, J., Zhang, S., Li, F., He, W., Labuda, K., Song, Y., Peterbauer, A., Wolbank, S., Redl, H., Zhong, M., Cai, D., Zeng, L., & Pei, D. (2010). Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell, 6(1), 71–79.
Xu, X., Wang, Q., Long, Y., Zhang, R., Wei, X., Xing, M., Gu, H., & Xie, X. (2013). Stress-mediated p38 activation promotes somatic cell reprogramming. Cell Research, 23(1), 131–141.
Maherali, N., & Hochedlinger, K. (2009). Tgfbeta signal inhibition cooperates in the induction of iPSCs and replaces Sox2 and cMyc. Current Biology, 19(20), 1718–1723.
Ichida, J. K., Blanchard, J., Lam, K., Son, E. Y., Chung, J. E., Egli, D., Loh, K. M., Carter, A. C., di Giorgio, F. P., Koszka, K., Huangfu, D., Akutsu, H., Liu, D. R., Rubin, L. L., & Eggan, K. (2009). A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell, 5(5), 491–503.
Zhang, Y., et al. (2012). Small molecules, big roles -- the chemical manipulation of stem cell fate and somatic cell reprogramming. Journal of Cell Science, 125(Pt 23), 5609–5620.
Lin, T., Ambasudhan, R., Yuan, X., Li, W., Hilcove, S., Abujarour, R., Lin, X., Hahm, H. S., Hao, E., Hayek, A., & Ding, S. (2009). A chemical platform for improved induction of human iPSCs. Nature Methods, 6(11), 805–808.
Zhu, S., Li, W., Zhou, H., Wei, W., Ambasudhan, R., Lin, T., Kim, J., Zhang, K., & Ding, S. (2010). Reprogramming of human primary somatic cells by OCT4 and chemical compounds. Cell Stem Cell, 7(6), 651–655.
Wang, W., et al. (2011). Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proceedings of the National Academy of Sciences of the United States of America, 108(45), 18283–18288.
Chen, T., Shen, L., Yu, J., Wan, H., Guo, A., Chen, J., Long, Y., Zhao, J., & Pei, G. (2011). Rapamycin and other longevity-promoting compounds enhance the generation of mouse induced pluripotent stem cells. Aging Cell, 10(5), 908–911.
Yuan, X., et al. (2011). Brief report: Combined chemical treatment enables Oct4-induced reprogramming from mouse embryonic fibroblasts. Stem Cells, 29(3), 549–553.
Li, Y., Zhang, Q., Yin, X., Yang, W., du, Y., Hou, P., Ge, J., Liu, C., Zhang, W., Zhang, X., Wu, Y., Li, H., Liu, K., Wu, C., Song, Z., Zhao, Y., Shi, Y., & Deng, H. (2011). Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Research, 21(1), 196–204.
Zhao, Y., Zhao, T., Guan, J., Zhang, X., Fu, Y., Ye, J., Zhu, J., Meng, G., Ge, J., Yang, S., Cheng, L., du, Y., Zhao, C., Wang, T., Su, L., Yang, W., & Deng, H. (2015). A XEN-like state bridges somatic cells to Pluripotency during chemical reprogramming. Cell, 163(7), 1678–1691.
Ye, J., Ge, J., Zhang, X., Cheng, L., Zhang, Z., He, S., Wang, Y., Lin, H., Yang, W., Liu, J., Zhao, Y., & Deng, H. (2016). Pluripotent stem cells induced from mouse neural stem cells and small intestinal epithelial cells by small molecule compounds. Cell Research, 26(1), 34–45.
Li, D., Wang, L., Hou, J., Shen, Q., Chen, Q., Wang, X., du, J., Cai, X., Shan, Y., Zhang, T., Zhou, T., Shi, X., Li, Y., Zhang, H., & Pan, G. (2016). Optimized approaches for generation of integration-free iPSCs from human urine-derived cells with small molecules and autologous feeder. Stem Cell Reports, 6(5), 717–728.
Lin, T.X. and S.H. Wu, Reprogramming with small molecules instead of exogenous transcription factors. Stem Cells International, 2015.
Li, X., et al. (2017). Direct reprogramming of fibroblasts via a chemically induced XEN-like state. Cell Stem Cell,21(2), 264.
Han, X., Yu, H., Huang, D., Xu, Y., Saadatpour, A., Li, X., Wang, L., Yu, J., Pinello, L., Lai, S., Jiang, M., Tian, X., Zhang, F., Cen, Y., Fujiwara, Y., Zhu, W., Zhou, B., Zhou, T., Ouyang, H., Wang, J., Yuan, G. C., Duan, S., Orkin, S. H., & Guo, G. (2017). A molecular roadmap for induced multi-lineage trans-differentiation of fibroblasts by chemical combinations. Cell Research, 27(3), 386–401.
Takeda, Y., et al. (2018). Chemical compound-based direct reprogramming for future clinical applications. Bioscience Reports, 38(3).
Li, X., Zuo, X., Jing, J., Ma, Y., Wang, J., Liu, D., Zhu, J., du, X., Xiong, L., du, Y., Xu, J., Xiao, X., Wang, J., Chai, Z., Zhao, Y., & Deng, H. (2015). Small-molecule-driven direct reprogramming of mouse fibroblasts into functional neurons. Cell Stem Cell, 17(2), 195–203.
Zhang, M. L., et al. (2016). Pharmacological reprogramming of fibroblasts into neural stem cells by signaling-directed transcriptional activation. Cell Stem Cell, 18(5), 653–667.
Fujii, Y., et al. (2019). The direct reprogramming of retinal astrocytes into neurons with small-molecule compounds. Investigative Ophthalmology & Visual Science, 60(9).
Lee, C., Robinson, M., & Willerth, S. M. (2018). Direct reprogramming of Glioblastoma cells into neurons using small molecules. ACS Chemical Neuroscience, 9(12), 3175–3185.
Hu, W. X., et al. (2015). Direct conversion of Normal and Alzheimer's disease human fibroblasts into neuronal cells by small molecules. Cell Stem Cell, 17(2), 204–212.
Pournasr, B., Asghari-Vostikolaee, M. H., & Baharvand, H. (2015). Transcription factor-mediated reprograming of fibroblasts to hepatocyte-like cells. European Journal of Cell Biology, 94(12), 603–610.
Lim, K. T., Lee, S. C., Gao, Y., Kim, K. P., Song, G., An, S. Y., Adachi, K., Jang, Y. J., Kim, J., Oh, K. J., Kwak, T. H., Hwang, S. I., You, J. S., Ko, K., Koo, S. H., Sharma, A. D., Kim, J. H., Hui, L., Cantz, T., Schöler, H. R., & Han, D. W. (2016). Small molecules facilitate single factor-mediated hepatic reprogramming. Cell Reports, 15(4), 814–829.
Kim, Y., Kang, K., Lee, S. B., Seo, D., Yoon, S., Kim, S. J., Jang, K., Jung, Y. K., Lee, K. G., Factor, V. M., Jeong, J., & Choi, D. (2019). Small molecule-mediated reprogramming of human hepatocytes into bipotent progenitor cells. Journal of Hepatology, 70(1), 97–107.
Lalit, P. A., Salick, M. R., Nelson, D. O., Squirrell, J. M., Shafer, C. M., Patel, N. G., Saeed, I., Schmuck, E. G., Markandeya, Y. S., Wong, R., Lea, M. R., Eliceiri, K. W., Hacker, T. A., Crone, W. C., Kyba, M., Garry, D. J., Stewart, R., Thomson, J. A., Downs, K. M., Lyons, G. E., & Kamp, T. J. (2016). Lineage reprogramming of fibroblasts into proliferative induced cardiac progenitor cells by defined factors. Cell Stem Cell, 18(3), 354–367.
Zhang, Y., Cao, N., Huang, Y., Spencer, C. I., Fu, J. D., Yu, C., Liu, K., Nie, B., Xu, T., Li, K., Xu, S., Bruneau, B. G., Srivastava, D., & Ding, S. (2016). Expandable cardiovascular progenitor cells reprogrammed from fibroblasts. Cell Stem Cell, 18(3), 368–381.
Kota, P. S., et al. (2017). Less may be more: Using small molecules to reprogram human cells into functional cardiomyocytes. The Journal of Thoracic and Cardiovascular Surgery, 153(1), 128–130.
Wang, Y. F., et al. (2016). Conversion of human gastric epithelial cells to multipotent endodermal progenitors using defined small molecules. Cell Stem Cell, 19(4), 449–461.
Zhu, S. Y., et al. (2016). Human pancreatic beta-like cells converted from fibroblasts. Nature Communications, 7.
Koblas, T., Leontovyc, I., Loukotová, S., & Saudek, F. (2019). Reprogramming of human pancreatic Organoid cells into insulin-producing beta-like cells by small molecules and in vitro transcribed modified mRNA encoding Neurogenin 3 transcription factor. Folia Biologica, 65(3), 109–123.
Takahashi, K., & Yamanaka, S. (2016). A decade of transcription factor-mediated reprogramming to pluripotency. Nature Reviews. Molecular Cell Biology, 17(3), 183–193.
Mahoney, J. E., et al. (2019). Ips cells: Models towards cell-based therapies. Pediatric Pulmonology, 54, S320–S320.
Uchimura, T., Otomo, J., Sato, M., & Sakurai, H. (2017). A human iPS cell myogenic differentiation system permitting high-throughput drug screening. Stem Cell Research, 25, 98–106.
Eglen, R. M., & Reisine, T. (2019). Human iPS cell-derived patient tissues and 3D cell culture part 2: Spheroids, Organoids, and disease modeling. SLAS Technol, 24(1), 18–27.
Hanna, J., Wernig, M., Markoulaki, S., Sun, C. W., Meissner, A., Cassady, J. P., Beard, C., Brambrink, T., Wu, L. C., Townes, T. M., & Jaenisch, R. (2007). Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science, 318(5858), 1920–1923.
Huang, X., Wang, Y., Yan, W., Smith, C., Ye, Z., Wang, J., Gao, Y., Mendelsohn, L., & Cheng, L. (2015). Production of gene-corrected adult Beta globin protein in human erythrocytes differentiated from patient iPSCs after genome editing of the sickle point mutation. Stem Cells, 33(5), 1470–1479.
Tsuji, O., Miura, K., Okada, Y., Fujiyoshi, K., Mukaino, M., Nagoshi, N., Kitamura, K., Kumagai, G., Nishino, M., Tomisato, S., Higashi, H., Nagai, T., Katoh, H., Kohda, K., Matsuzaki, Y., Yuzaki, M., Ikeda, E., Toyama, Y., Nakamura, M., Yamanaka, S., & Okano, H. (2010). Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America, 107(28), 12704–12709.
Pereira, I. M., et al. (2019). Filling the gap: Neural stem cells as a promising therapy for spinal cord injury. Pharmaceuticals, 12(2).
Kriks, S., Shim, J. W., Piao, J., Ganat, Y. M., Wakeman, D. R., Xie, Z., Carrillo-Reid, L., Auyeung, G., Antonacci, C., Buch, A., Yang, L., Beal, M. F., Surmeier, D. J., Kordower, J. H., Tabar, V., & Studer, L. (2011). Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature, 480(7378), 547–U177.
Takagi, R., et al. (2016). Bioengineering a 3D integumentary organ system from iPS cells using an in vivo transplantation model. Science Advances, 2(4).
Charles, J., et al. (2019). Multi-organ failure induced by Nivolumab in the context of Allo-stem cell transplantation. Experimental Hematology & Oncology, 8.
Macas, S. C., et al. (2019). Generation and differentiation of an optogenetic human neural stem cell for Parkinson's disease. Human Gene Therapy, 30(11), A105–A105.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No.81802960), the Fundamental Research Funds for the Central Universities (No.22120180035), the Shanghai Municipal Population and Family Planning Commission (No.201640346), the National Natural Science Foundation of China (No.81671468), Shanghai Clinical Medicine Field Project of Science and Technology Innovation Action Program (No.17411951600) and Shanghai Municipal Medical and Health Discipline Construction Projects (No.2017ZZ02015).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
There are no conflicts of interest in the studies reported in the paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, G., Guo, Y., Li, C. et al. Small Molecules that Promote Self-Renewal of Stem Cells and Somatic Cell Reprogramming. Stem Cell Rev and Rep 16, 511–523 (2020). https://doi.org/10.1007/s12015-020-09965-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-09965-w