Abstract
Progressive multifocal leukoencephalopathy (PML) is a viral disease of the brain associated with immunodeficiency, immune suppressing medications, and malignancy. In the absence of effective anti-viral therapy for the causative JC virus, immune restoration has emerged as the critical therapeutic alternative. The evolving treatment of PML (and other rare JC virus–associated neurologic syndromes) requires consideration of baseline immune functioning and comorbid diseases while selecting from a number of therapeutic options to restore an effective immune response. This review focuses on the current options for management of PML in typical situations where this disease presents, including several where immune restoration is a standard therapeutic approach such as in PML associated with HIV/AIDS and in multiple sclerosis associated with natalizumab. Other circumstances in which PML occurs including associated with primary immunodeficiencies, malignancies, and transplants present greater challenges to immune reconstitution, but emerging concepts may enhance therapeutic options for these situations. Particular attention is focused on recent experience with checkpoint inhibitors, guidance for MS drug discontinuation, and strategies to monitor and facilitate immune restoration.
Similar content being viewed by others
Progressive multifocal leukoencephalopathy (PML) is a rare, potentially lethal viral brain disease that has proved challenging to treat. The majority of adults are asymptomatically infected by the causative JC polyomavirus (JCV). The wild-type (archetype) virus found in healthy individuals remains asymptomatic in urogenital tissue even while replicating, causing no symptoms at acute infection or later. Long periods of immunosuppression appear to promote viral reactivation and unregulated replication resulting in viral transformation to a more pathologic or prototype virus prone to disseminate to the brain, cause lytic infection of oligodendrocytes, and demyelination that ultimately causes symptoms of PML [1]. Unfortunately, the serum JCV antibodies generated with chronic viral infection in a majority of adults do not prevent the development of PML [2,3,4]. As the central nervous system (CNS) infection becomes established, progression of neurological deficits is evidenced by white matter lesions on brain MRI scans. Classic PML is identified pathologically by areas of demyelination, immunohistochemical staining of JC virus in the nuclei of astrocytic cells and oligodendroglia (see Fig. 1b, c), and bizarre large astrocytes transformed by viral infection. The typical lesion has little inflammation in the lesions and the blood–brain barrier remains intact. When immune reconstitution is accomplished, the lesions change with marked cellular inflammatory response led by CD8 cells. The common clinical setting for PML has shifted over the last four decades. While literature focused on HIV/AIDS-associated PML in the past, clinicians currently are more concerned about PML risk in the setting of immunomodulatory medications. This review covers therapeutic approaches to PML in each of the settings where it is encountered (Table 1). Clinical presentation, diagnostic considerations, and MRI features have been reviewed recently [1, 14].
(A) Low magnification H&E stain of the left parietal lobe lesion with a heterogeneous inflammatory cell infiltrate with areas of pallor corresponding to myelin loss. (B) The enlarged lightly “plum colored” nucleus (arrow) of an infected oligodendrocyte is compared to one of normal size (arrowhead). (C) Immunoreactivity of polyoma antigen further establishes the diagnosis of PML. (D, E) Immunohistochemical demonstration of CD4 and CD8 respectively (F). Plasma cells in the inflammatory process are demonstrated by CD138 staining
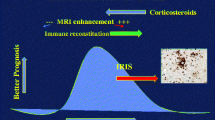
CD4 lymphocytes are required for JCV antigen recognition and signaling to CD8 cells, which, in turn, promote viral clearance. Treatment of PML must facilitate a CNS immune response with JCV specific cytotoxic CD8 T cells. Because this disease is subacute and progressive, delayed immune responses are associated with a worse prognosis as the virus continues a lytic infection with progressive brain injury. PML occurring with immune deficiencies that cannot be readily restored has a much worse prognosis due to progression of viral-driven brain damage. On the other hand, a robust influx of CD4 and numerous CD8 cells may precipitate a clinical decline not explained by the natural course of PML. This paradoxical deterioration is known as CNS immune reconstitution inflammatory syndrome (IRIS), a process that augments symptoms and potentially brain injury. While these deleterious responses could improve during recovery, balancing this immune restoration to promote viral clearance and minimize an excessive inflammatory process is the foundation for our current approach to PML treatment.
PML is associated with many types of immunodeficiency, malignancy, transplants, and medications [15], and specific anti-viral treatments for JCV have remained elusive. Researchers have failed to prove efficacy of DNA antivirals, targets such as mefloquine discovered by high-throughput drug screening, and blockage of JCV serotoninergic receptor binding on glial cells. The older approaches, reviewed previously in more detail [16], have included the unsuccessful trials of cytosine arabinoside [17], cidofovir [18, 19], and mefloquine [20, 21]. Mirtazapine [22] and antipsychotics have not been tested prospectively, but little evidence supports efficacy and they are not recommended as a routine.
Lacking means to specifically treat JCV, therapeutic efforts have focused on immune augmenting approaches. The options vary by disease setting, so approach to therapy will be discussed relevant to each of the major situations in which PML develops.
HIV/AIDS-Associated PML
Prior to effective therapy for AIDS, HIV-associated PML almost always resulted in death within months. Remarkably, combination antiretroviral treatment (cART) improved survival from 10% [23] to as high as 83% in some reports [24,25,26,27]. Those patients who survived the JC infection may have long life spans with modest disability and no expectation for PML recurrence [28]. PML survival appears largely dependent on control of systemic HIV, while CNS penetrance of the therapy is probably of no importance [29]. Specific cART backbones have not proven to be significantly different in PML survival [29, 30].
While CD4 counts are an excellent way to estimate the severity of immunodeficiency in AIDS, they are less reliable predictors of PML risk or outcomes than in many other co-infectious complications. PML is more prevalent in advanced HIV with lower CD4 counts [31], but the disease does occur occasionally with higher CD4 counts [32]. Immune reconstitution appears shortly after starting cART, even before CD4 counts substantially increase, making initiation of cART a surprisingly effective therapy for PML.
The majority of PML continues to be seen within the HIV population and is commonly diagnosed as a presenting AIDS diagnosis or within the first months of treatment. Not infrequently, PML in HIV is completely unrecognized until the initiation of cART, when the diagnosis becomes evident when presymptomatic disease worsens with the onset of PML-IRIS. As with IRIS seen in other opportunistic infections, prior severe immunodeficiency tends to be a harbinger for a more dramatic IRIS. In our experience, IRIS in the setting of HIV-associated PML tends to be less severe than in the natalizumab-treated MS cases, perhaps due to the time required to repair the HIV-damaged immune systems.
While HIV-associated PML is less frequently seen in an era with effective and early use of HIV treatments, HIV remains a common association with PML. PML certainly has the potential to be lethal, but in HIV this complication is remarkably treatable. Serious symptoms may be at least partially reversible if they are driven by IRIS rather than the more permanent damage the JC virus inflicts, making the immediate start of cART imperative. Because some improvement is possible, supportive care is justified in most HIV-associated PML patients, and every effort to quickly control HIV is critical.
Natalizumab-Associated PML
PML is an uncommon complication of some immune suppressing medications. Natalizumab, an integrin inhibitor used to treat multiple sclerosis (MS), has had the most troubled history in this regard. It blocks the entry of lymphocytes into the CNS and thus reduces the inflammatory activity of MS. Excitement generated by this effective disease-modifying MS therapy was quickly blunted when several cases of PML occurred shortly after licensing the drug [33]. The enhanced PML risk is likely due to the combination of reactivation of circulating virus from the bone marrow and partially inhibited immune surveillance of the brain. A higher risk of developing PML is associated with rising JCV antibody titers, longer durations of therapy, and previous immunosuppressant use. When all of the risks combine, MS patients on natalizumab have a risk of PML exceeding 1%. Strategies for mitigation of this risk have been discussed in several manuscripts [34, 35]. In low-risk groups with careful monitoring, the drug can be used safely for many years, but when risks increase and alternative therapies are available, it makes sense to stop natalizumab therapy. If PML develops on natalizumab, prompt discontinuation of this drug will achieve immune reconstitution and relatively high PML survival rates of 71–92% are reported [36,37,38,39,40]. The elevated risk of PML seems to exist for approximately 6 months after discontinuing natalizumab, but lifelong elevated risk has not been suggested thus far.
There remains some controversy about whether additional measures should be undertaken when PML is discovered in a patient still taking natalizumab. The long duration of action argues if the last natalizumab infusion has been within a month or two for use of plasma exchange to speed the elimination of the drug [41]. Some retrospective case studies suggest little value for plasma exchange, leaving open discussion around this point [42,43,44]. Our practice has been to recommend use of plasma exchange given the rapidity with which symptomatic PML progresses, and lack of evidence that delayed drug removal avoids IRIS or prevents brain injury [40]. Our view remains that IRIS is likely in any case, at least for severe cases of PML. Plasma exchange has been used in a majority of the most serious cases of natalizumab-associated PML, and the relatively favorable survival supports this practice. In less extensive disease, plasma exchange is not required. The concern that severe IRIS may develop with sudden elimination of natalizumab and might be detrimental to patient would require a prospective study to determine.
Another controversial topic is the decision to use corticosteroids when faced with PML and IRIS. Again, prospective studies are not available, and the theoretical concerns about use of corticosteroids overall are not substantiated by the relatively successful outcomes with liberal use of this approach to mitigate IRIS. That said, the need for corticosteroids is variable. The authors currently recommend waiting until clinical progression occurs following immune reconstitution efforts, then using intermittent IV steroid infusions to attenuate clinical expressions of IRIS. Alternatives to corticosteroids for preventing or treating natalizumab PML IRIS have not been adequately studied. Case series suggest some utility for maraviroc [45]. However, in many cases, these measures are not required.
Autoimmune Disease and Medication-Associated PML
Many other medications used in autoimmune diseases, rheumatologic disease, and in treatment of malignancy are also associated with PML. Because each of these diseases may have an intrinsic risk for PML, these associations are often difficult to disentangle from the risk of underlying disease. Fingolimod [46], B cell–depleting therapies such as rituximab [47,48,49], fumaric acid esters [50,51,52,53], and others [28, 54,55,56,57] have been associated with a low but real added risk of PML. In most cases, prospective risk determination is futile, and the cases are so rare that proof of any strategy proposed would be impractical. However, long-term lymphopenia is likely a marker of some increased risk. In the case of fumarates, lymphopenia is seen in most of the PML cases, and recommendations to monitor lymphopenia have been adopted. Discontinuation of therapy with prolonged lymphopenia < 500 cells is likely to reduce the small risk of fumarates further, but will not prevent all cases of PML [53, 58].
When PML occurs on therapy with one of these immune suppressing drugs in autoimmune diseases, simply decreasing the degree of immunosuppression by discontinuing the suspect drug or substantially reducing immune suppressing combination therapy is sometimes sufficient to stimulate immune reconstitution. Many of these drugs have a sufficiently short duration of action to be amenable to rapid reversibility. When inflammation ensues, similar approaches to symptomatic treatment of IRIS with corticosteroids are sometimes required.
Not all of the drugs used in this setting are rapidly reversible. The monoclonal antibody B cell antagonists cause long-term loss of B cells, and once this change is established, plasma exchange has no place in therapy. Fortunately, risk of PML in this setting is rare, and with limitation of other immune suppressing agents, survival remains possible even in the absence of an expeditious B cell improvement. Ocrelizumab is a recently introduced B cell–depleting therapy used in MS therapy and thus far also appears to have little additional risk for PML when used in MS patients. Indeed, it is striking that PML cases encountered after switching from natalizumab to ocrelizumab have had surprisingly low mortality [59].
The imprecision of monitoring circulating CD4 cells as a risk for PML is starkly illustrated by experience with alemtuzumab. This CD52 monoclonal has very long duration suppression of T cells following infusion. Remarkably, alemtuzumab has only had one attributable case of PML [54] in use for MS, despite the pronounced suppression of T and B cells with median recoveries of CD8 and CD4 T cells at 20 and 35 months respectively [60].
Therapy for PML in the setting of irreversible therapies such as alemtuzumab or ocrelizumab is largely supportive. For monoclonal therapies with long duration of action, plasma exchange rarely should be considered as it will be futile. Depending on the course, many physicians add safer adjunctive therapies that do not have the support of clinical trials but may still have some efficacy that has been difficult to discern. Mirtazapine or mefloquine are frequently used by clinicians. Mirtazapine is a rational antidepressant or sleep aid with a theoretical mechanism that might further inhibit PML, albeit without clinical evidence substantiating that potential. Mefloquine similarly has in vitro evidence suggesting possible efficacy, and while the clinical trial failed to document efficacy in a cohort with overall good outcomes, some benefit still might be added when no other safe therapeutic alternatives are available. Neither is recommended as routine therapy when avenues to immune restoration are clearly available. For PML patients with a progressive course not believed to be due to IRIS, research efforts to enhance T cell recovery as discussed subsequently in this review could be considered.
Hematologic Malignancy– and Solid Organ Transplantation–Associated PML
PML occurring in the setting of primary immunodeficiencies, hematologic malignancies, and organ transplantation has substantially worse prognosis than the above conditions. The comparatively high PML survival rate in the cohorts of PML where effective immune reconstitution is regularly attained has bolstered the ideas of actively fostering immune reconstitution. In the case of PML associated with solid organ transplants, clinicians must walk the line of decreased immune suppression threatening loss of the organ, or simply sacrificing the transplanted organ if it is non-essential. Sadly, often neither approach is successful [61].
Of particular interest is PML associated with hematologic malignancies, a setting where the underlying disease is difficult to reverse and treatment often requires further damage to the immune systems. If the underlying immune system remains severely impaired, PML usually results in death before an effective immune reconstitution can be achieved. Additional measures to augment immune reconstitution, considered below, may be of special interest in these patients.
Checkpoint Inhibitors for PML?
Several groups are exploring mechanisms to facilitate immune recognition using checkpoint inhibitors in the treatment of PML. Checkpoint receptors, such as programmed cell death-1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA4), are immune inhibitory. The upregulation of these inhibitory receptors is a mechanism by which the JCV and other viruses evade the effector limb of the immune system [62, 63]. Researchers hypothesized that blocking these receptors shifted a tolerant or exhausted T cell population back towards lymphocyte proliferation and clearance of virus [62].
A promising report of long-term survival of a patient with PML in the setting of hematologic malignancy treated with nivolumab [64] was shortly followed by a case series on PML patients treated with pembrolizumab [5, 6] and nivolumab [7], both antibodies to PD-1, suggesting stabilization or improvement in 8 of 11 patients. Paraclinical markers reported were also supportive of survival [65]. Since malignancy- and solid organ transplant–associated PML patients have poor outcomes, the PD-1 inhibitors’ success supports consideration as a treatment in this setting [61, 66, 67]. However, as subsequent cases are reported, the evidence indicates this is not a transformative therapy for PML [68,69,70]. The risks of these drugs are substantial, and when immune reconstitution can be accomplished without them, we do not recommend their use. These drugs remain a plausible approach to enhance immune recovery when other approaches are unavailable.
Another approach is use of IL-7 or IL-2 therapy to enhance cellular immune responses required to survive PML. The rationale behind IL-7 use in chronic viremia was reviewed [71, 72], and cases reported [11,12,13, 73, 74] have suggested a boost to lymphoid cell response. IL-2, which can promote T effector cell differentiation, has also been tried for PML with hopes that it may enhance control of JC virus [75,76,77]. Unfortunately, IL-2 delivery has been limited by significant side effects and has not appeared effective. Lastly, filgrastim, known as granulocyte colony-stimulating factor, promotes the production of antigen presenting cells, specifically granulocytes, and has been important in the management of neutropenia. The potential role for filgrastim in PML treatment seems limited to a few examples of poor antigen recognition or myeloid lineage deficiencies and remains to be explored [43].
Allogeneic t Cell Therapy
A recent report using ex vivo expanded allogeneic BK-specific T cell infusions for PML patients illustrates a novel approach to cellular immune reconstitution [10]. Adoptive transfer of BK-specific T cells has been used in treatment of BK infections. Since the BK virus shares substantial homology with JCV, the possibility that infusions of BK targeted lymphocytes may be an effective treatment for PML seemed plausible. The reported reduction in JC viral load associated with clinical improvement in 1 of the 2 non-HIV patients was promising. An additional case was alluded to in a review of cases reported by Anand et al. [27]. The preparation, storage, and later use makes this an attractive potential therapy. While not representing a practical clinical approach at most centers presently, this is an area for further research and may be of particular interest for PML patients with a poor ability to mount an immune response. As of now, we cannot draw conclusions about clinical use. Side effects and long-term survival will be better appreciated with additional experience.
Vaccines and Other Potential Therapies for JC Virus
A different mechanism for approaching JC virus infections is with immunization [36, 78]. On initial consideration, antibodies seem well documented as failing to prevent PML, and indeed the disease occurs in the face of high and rising antibodies, as seen with natalizumab. However, PML may occur in the setting of mismatched neutralizing antibodies as the JC virus mutates epitopes and becomes neurotropic. Thus, further humoral stimulation could elicit an effective neutralizing antibody response contributing to control of PML [79]. Active immunization research proposes using various vaccines for prophylaxis (or potentially treatment) against PML by formulating immunogenic JCV antigens, such as the major structural capsid protein VP1. Providing anti-JCV VP-1 antibodies for passive neutralization is another area being explored, and antibodies seem to be able to bind both the archetype and VP1 mutants associated with PML. There has been some use of IVIG in PML that might have validity if these concepts are true. At this time, however, evidence fails to support practical efficacy for these approaches. Lastly, considerations of genetic engineering such as made possible by CRISPR/Cas9 system are also being considered and will no doubt be an area for future investigation [80].
Challenges and Opportunities of Immune Reconstitution
PML too often leaves serious neurologic injuries even when the JC disease is controlled. Emerging experience makes it clear that survival is possible, and underlying brain injury may be modest in fortunate patients. Our experience confronting JC virus and PML suggests even modest improvement in immunity is often sufficient to achieve long-term (permanent) control of the JCV. Reliance on immune reconstitution challenges the clinician to manage this therapeutic exercise as safely as possible. The marked clinical and MRI worsening that occur during IRIS are concerning to patients and clinicians. We believe IRIS is unavoidable in treating PML and should not be delayed because delay leads to generation of additional JC antigen and accumulation of permanent brain injury from JCV. The worsening seen during IRIS is often at least partially reversible over the following 6–12 months during recovery. Slowing or delaying the immune recovery is not recommended, although prospective studies to compare differing approaches to immune reconstitution have not been possible.
PML-IRIS response can be impossible to distinguish from PML progression and current tools quantify it poorly. Most clinicians rely on MRI lesion enhancement as an indicator of inflammatory PML. While this is a good indicator of severe IRIS, it does not appear to be sensitive [81]. Clinically significant IRIS has been documented in pathologic samples of brain without noticeable gadolinium-enhancing lesions on MRI. We believe early PML IRIS may be recognized by T2 bright punctate perivascular lesions on MRI (Fig. 2) [82], pathologically correlating with abundant inflammatory cells infiltrating in perivascular distribution as well as PML lesions (Fig. 1a, d–f). Noting a “starry sky” configuration may help the clinician be more secure in believing that immune reconstitution is underway, and that treatment for IRIS is likely appropriate. Figures 1 and 2 illustrate such a case occurring in a patient with untreated sarcoidosis with lymphopenia in whom PML had developed, and the inflammatory PML lesions presented a “starry sky” distribution on MRI. In contrast, severe PML IRIS may have large expanded lesions with mass effect [83], although this degree of response is uncommon.
(A) MRI brain axial T2 FLAIR with large left parietal and smaller subcortical white matter lesions with (B) subtle punctate T1 gadolinium enhancement (arrows) in early PML IRIS. The T2 lesions generally preceded a more prominent response (C) 1 month later. The axial T2 FLAIR showed mild progression of white matter disease and (D) prominent punctate enhancement known as the “starry sky” of full PML IRIS on the T1 sequence
Our practice is to use corticosteroids for IRIS in response to clinical rather than imaging changes. Sometimes MRI worsening during IRIS is visually dramatic whereas the patient is unchanged or even slightly improved. It has seemed prudent to respond therapeutically to clinical decline, and not rely on MRI imaging for therapeutic decisions. Clinicians should avoid the mistake of assuming an inflammatory response requires demonstration of MRI enhancement [81]. A dramatic clinical decline in the weeks after immune reconstitution should prompt clinicians to suspect IRIS and manage with courses of corticosteroids. Our experience suggests that corticosteroids do not enhance the danger of JCV infections substantially, but can blunt the symptomatic and dangerous IRIS responses that typically are present. Steroids may be lifesaving in managing the unwanted degree of injury seen occasionally with IRIS. Additionally, the clinical responsiveness to steroids is a crude, albeit helpful, indicator of IRIS, particularly considering the insensitive MRI measures available for following the clinical course.
Finally, it is important to realize that the IRIS response continues for months, and that periodic repeat courses of steroids are not uncommonly required. While these clinical relapses are difficult to distinguish from JCV relapse or MS exacerbation, pathologic documentation of ongoing PML IRIS has been seen at least 5 months following natalizumab withdrawal. We recommend starting therapy with methylprednisolone 1 g IV for 3–5 days with or without individual tapering oral prednisone dosing. Additional courses may well be required several times at approximately monthly intervals.
The other concern unique to PML in the setting of multiple sclerosis is the challenge of distinguishing an MS exacerbation (which sometimes is severe during therapy withdrawal) from PML with IRIS [84,85,86,87,88]. Rebound MS-related inflammation can be particularly severe with natalizumab and fingolimod [89] withdrawal, and should also prompt consideration for steroid use.
A complex part of multiple sclerosis drug management is the approach to carry over cases of PML from prior natalizumab therapy. Patients requiring therapy switches from natalizumab have gone on to a diagnosis of PML within several months of starting ocrelizumab [59] and fingolimod [90], among others. Remarkably, PML survival has been very good in carry-over cases with subsequent ocrelizumab exposure [59]. These early reports are encouraging that B cell–directed therapy like rituximab and ocrelizumab will not carry disproportionate risk. In addition, restarting MS treatment following resolution of PML seems to have little danger of PML recurrence [43]. With a substantial number of disease-modifying treatments available for multiple sclerosis, there are many choices for subsequent medications to transition high-risk patients. Clinicians have little empirical guidance for choosing the next disease-modifying therapy for MS, and choices should be made collaboratively with the patients based on broad understanding of risks and benefits of the individual therapies.
Alternative approaches to prevent or control IRIS in PML would be valuable. A report in 2009 described a quick recovery from PML-IRIS in HIV using maraviroc [91] and suggested the CCR5 inhibitor used in HIV treatment could reduce CCR5+ immune cells from trafficking into the brain. Subsequently, a multicenter trial demonstrated no significant benefit with maraviroc in preventing IRIS in HIV patients started on cART, although PML was not specifically studied [92]. Additional support for CCR5 modulation with PML IRIS demonstrated high levels of CCR5 expression in natalizumab-associated IRIS [93, 94]. Currently, there is not a clear role for maraviroc in PML IRIS but further evaluation is required [95].
Summary
PML is the most common JC virus pathologic manifestation, but other variations of JC virus human disease have recently been described including JC granular cell neuronopathy, meningitis, and encephalitis that occur with similar predisposing circumstances. Efforts to treat these might reasonably include similar tactics to those we outline for PML.
The treatment of PML should consider the immune conditions leading to vulnerability and seek to reverse these. Survival improves with declining CSF JCV DNA loads as the replicative rates are controlled and the immune system facilitates viral clearance [31, 96, 97]. Profound impairment of the cellular immune system or expectation for long-lasting T cell dysfunction after medication discontinuation is considered a poor prognostic sign, and experimental approaches to enhance immunity such as PD-1 inhibitors, interleukins, growth factors, or adoptive polyomavirus-specific T cell transfers might be tested most effectively in this setting. For many situations where PML is encountered, there is an excellent chance of combatting this formerly fatal complication. Testing experimental therapies in settings with an expected rapid immune recovery and high survival rate have little chance of informing researchers on the value of interventions, while subjecting these patients to additional risks and cost. These should be approached with reluctance.
Recognizing that PML IRIS will occur as immune reconstitution is achieved and that it may require modulation for optimal outcomes remains a critical challenge for clinicians treating PML. PML-IRIS management with corticosteroids is an important part of modulating the intensity of the immune response to PML when clinically dangerous features of IRIS are present [98]. With careful management, PML can be survived. Rapid diagnosis and skilled management can result in more favorable long-term survival.
References
Major EO, Yousry TA, Clifford DB. Pathogenesis of progressive multifocal leukoencephalopathy and risks associated with treatments for multiple sclerosis: a decade of lessons learned. Lancet Neurol 2018;17(5):467–80.
Weber T, Trebst C, Frye S, Cinque P, Vago L, Sindic CJM, et al. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J Infect Dis 1997;176:250–4.
Knowles WA, Pipkin P, Andrews N, Vyse A, Minor P, Brown WG, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol 2003;71:115–23.
Bozic C, Richman S, Plavina T, Natarajan A, Scanlon JV, Subramanyam M, et al. Anti-John Cunnigham virus antibody prevalence in multiple sclerosis patients: Baseline results of STRATIFY-1. Ann Neurol 2011;70(5):713–21.
Rauer S, Marks R, Urbach H, Warnatz K, Nath A, Holland S, et al. Treatment of Progressive Multifocal Leukoencephalopathy with Pembrolizumab. N Engl J Med 2019;380(17):1676–7.
Cortese I, Muranski P, Enose-Akahata Y, Ha SK, Smith B, Monaco M, et al. Pembrolizumab Treatment for Progressive Multifocal Leukoencephalopathy. NEJM 2019;380(17):1597–605.
Walter O, Treiner E, Bonneville F, Mengelle C, Vergez F, Lerebours F, et al. Treatment of Progressive Multifocal Leukoencephalopathy with Nivolumab. NEJM 2019;380(17):1674–6.
Hoang E, Bartlett NL, Goyal MS, Schmidt RE, Clifford DB. Progressive multifocal leukoencephalopathy treated with nivolumab. J Neurovirol 2019.
Darvin P, Toor SM, Sasidharan NV, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med 2018;50(165).
Muftuoglu M, Olson A, Marin D, Ahmed S, Mulanovich V, Tummala S, et al. Allogeneic BK Virus-Specific T Cells for Progressive Multifocal Leukoencephalopathy. N Engl J Med 2018;379(15):1443–51.
Patel A, Patel J, Ikwuagwu J. A case of progressive multifocal leukoencephalopathy and idiopathic CD4+ lymphocytopenia. J Antimicrob Chemother 2010;65(12):2697–8.
Gasnault J, de Goer de Herve MG, Michot JM, Hendel-Chavez H, Seta V, Mazet AA, et al. Efficacy of recombinant human interleukin 7 in a patient with severe lymphopenia-related progressive multifocal leukoencephalopathy. Open Forum Infect Dis. 2014;1(2):ofu074.
Alstadhaug KB, Croughs T, Henriksen S, Leboeuf C, Sereti I, Hirsch HH, et al. Treatment of progressive multifocal leukoencephalopathy with interleukin 7. JAMA Neurol 2014;71(8):1030–5.
Berger JR, Aksamit AJ, Clifford DB, Davis L, Koralnik IJ, Sejvar JJ, et al. PML diagnostic criteria: Consensus statement from the AAN Neuroinfectious Disease Section. Neurology 2013;80(15):1430–8.
Brooks BR, Walker DL. Progressive multifocal leukoencephalopathy. Neurol Clin 1984;2(2):299–313.
Clifford DB. Progressive multifocal leukoencephalopathy therapy. J Neurovirol 2015;21(6):632–6.
Hall CD, Dafni U, Simpson D, Clifford DB, Wetherill PE, Cohen B, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. NEnglJMed 1998;338:1345–51.
Marra CM, Rajicic N, Barker DE, Cohen BA, Clifford D, Post MJD, et al. A pilot study of cidofovir for progressive multifocal leukoencephalopathy in AIDS. AIDS 2002;16:1–7.
Gasnault J, Kousignian P, Kahraman M, Rahoiljaon J, Matheron S, Delfraissy JF, et al. Cidofovir in AIDS-associated progressive multifocal leukoencephalopathy: a monocenter observational study with clinical and JC virus load monitoring. J Neurovirol 2001;7(4):375–81.
Clifford DB, Nath A, Cinque P, Brew BJ, Zivadinov R, Gorelik L, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol 2013;19(4):351–8.
Brickelmaier M, Lugovskoy A, Kartikeyan R, Reviriego-Mendoza MM, Allaire N, Simon K, et al. Identification and characterization of mefloquine efficacy against JC virus in vitro. Antimicrob Agents Chemother 2009;53(5):1840–9.
Jamilloux Y, Kerever S, Ferry T, Broussolle C, Honnorat J, Seve P. Treatment of Progressive Multifocal Leukoencephalopathy With Mirtazapine. Clin Drug Investig 2016;36(10):783–9.
Berger JR, Pall L, Lanska D, Whiteman M. Progressive multifocal leukoencephalopathy in patients with HIV infection. J Neurovirol 1998;4:59–68.
Engsig FN, Hansen AB, Omland LH, Kronborg G, Gerstoft J, Laursen AL, et al. Incidence, clinical presentation, and outcome of progressive multifocal leukoencephalopathy in HIV-infected patients during the highly active antiretroviral therapy era: a nationwide cohort study. J Infect Dis 2009;199(1):77–83.
Falco V, Olmo M, Villar del Saz S, Guelar A, Santos JR, Gutierrez M, et al. Influence of HAART on the clinical course of HIV-1-infected patients with progressive multifocal leukoencephalopathy: results of an observational multicenter study. J Acquir Immune Defic Syndr. 2008;49:26–31.
Dworkin MS, Wan PT, Hanson DL, Jones JL, Project AASoHD. Progressive multifocal leukoencephalopathy: improved survival of human immunodeficiency virus-infected patients in the protease inhibitor era. J Infect Dis 1999;180:621–5.
Clifford DB, Team NARCS. Natural history of progressive multifocal leukoencephalopathy (PML) in AIDS modified by antiretroviral therapy. J Neurovirol 1998;4:346.
Anand P, Hotan G, Vogel A, Venna N, Mateen FJ. Progressive multifocal leukoencephalopathy A 25-year retrospective cohort study. Neurol Neuroimmunol Neuroinflamm. 2019;6(6).
Lanoy E, Guiguet M, Bentata M, Rouveix E, Dhiver C, Poizot-Martin I, et al. Survival after neuroAIDS: Association with antiretroviral CNS Penetration-Effectiveness score. Neurology 2011;76(7):644–51.
Fanjul F, Riveiro-Barciela M, Gonzalez J, Delgado E, Murillas J, Payeras Cifre A, et al. Evaluation of progressive multifocal leukoencephalopathy treatments in a Spanish cohort of HIV-infected patients: do protease inhibitors improve survival regardless of central nervous system penetration-effectiveness (CPE) score? HIV Med 2013;14(5):321–5.
Bossolasco S, Calori G, Moretti F, Boschini A, Bertelli D, Mena M, et al. Prognostic significance of JC virus DNA levels in cerebrospinal fluid of patients with HIV-associated progressive multifocal leukoencephalopathy. Clin Infect Dis 2005;40(5):738–44.
Gheuens S, Bord E, Kesari S, Simpson D, Gandhi R, Clifford D, et al. Combined role of CD4+ and CD8+ T cell responses against JCV in the clinical outcome of patients with PML and PML-IRIS. 18th Conference on Retroviruses and Opportunistic Infections. 2011;416.
Yousry TA, Major EO, Ryschkewitsch C, Fahle G, Fischer S, Hou J, et al. Evaluation of patients treated with natalizumab for progressive multifocal leukoencephalopathy. N Engl J Med 2006;354:924–33.
McGuigan C, Craner M, Guadagno J, Kapoor R, Mazibrada G, Molyneux P, et al. Stratification and monitoring of natalizumab-associated progressive multifocal leukoencephalopathy risk: recommendations from an expert group. J Neurol Neurosurg Psychiatry 2016;87(2):117–25.
Ho PR, Koendgen H, Campbell N, Haddock B, Richman S, Chang I. Risk of natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: a retrospective analysis of data from four clinical studies. Lancet Neurol 2017.
Pavlovic D, Patera AC, Nyberg F, Gerber M, Liu M, Progressive Multifocal Leukeoncephalopathy C. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord 2015;8(6):255–73.
Dong-Si T, Gheuens S, Gangadharan A, Wenten M, Philip J, McIninch J, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol 2015.
Chalkley JJ, Berger JR. Progressive multifocal leukoencephalopathy in multiple sclerosis. Curr Neurol Neurosci Rep. 2013;13(408).
Clifford DB, DeLuca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 2010;9(4):438–46.
Prosperini L, de Rossi N, Scarpazza C, Moiola L, Cosottini M, Gerevini S, et al. Natalizumab-Related Progressive Multifocal Leukoencephalopathy in Multiple Sclerosis: Findings from an Italian Independent Registry. PLoS ONE. 2016;11(12).
Khatri BO, Man S, Giovannoni G, Koo AP, Lee JC, Tucky B, et al. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology 2009;72:402–9.
Scarpazza C, Prosperini L, De Rossi N, Moiola L, Sormani MP, Gerevini S, et al. To do or not to do? Plasma exchange and timing of steroid administration in PML. Ann Neurol. 2017.
Stefoski D, Balabanov R, Waheed R, Ko M, Koralnik IJ, Sierra Morales F. Treatment of natalizumab-associated PML with filgrastim. Ann Clin Transl Neurol 2019;6(5):923–31.
Landi D, De Rossi N, Zagaglia S, Scarpazza C, Prosperini L, Albanese M, et al. No evidence of beneficial effects of plasmapheresis in natalizumab-associated PML. Neurology 2017;88(12):1144–52.
Giacomini PS, Rozenberg A, Metz I, Araujo D, Arbour N, Bar-Or A, et al. Maraviroc and JC virus-associated immune reconstitution inflammatory syndrome. N Engl J Med 2014;370(5):486–8.
Berger JR, Cree BA, Greenberg B, Hemmer B, Ward BJ, Dong VM, et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology 2018;90(20):e1815-e21.
Harris HE. Progressive multifocal leucoencephalopathy in a patient with systemic lupus erythematosus treated with rituximab. Rheumatology 2008;47(2):224–5.
Pejsa V, Lucijanic M, Jonjic Z, Prka Z, Vukorepa G. Progressive multifocal leukoencephalopathy developing after obinutuzumab treatment for chronic lymphocytic leukemia. Ann Hematol 2019;98(6):1509–10.
Berger JR, Malik V, Lacy S, Brunetta P, Lehane PB. Progressive multifocal leukoencephalopathy in rituximab-treated rheumatic diseases: a rare event. J Neuro-Oncol 2018;24(3):323–31.
Ermis U, Weis J, Schulz JB. PML in a patient treated with fumaric acid. N Engl J Med 2013;368(17):1657–8.
Ermis U, Weis J, Schulz JB. Case reports of PML in patients treated for psoriasis. N Engl J Med 2013;369(11):1081.
van Oosten BW, Killestein J, Barkhof F, Polman CH, Wattjes MP. PML in a patient treated with dimethyl fumarate from a compounding pharmacy. N Engl J Med 2013;368(17):1658–9.
Nieuwkamp DJ, Murk JL, van Oosten BW, Cremers CH, Killestein J, Viveen MC, et al. PML in a patient without severe lymphocytopenia receiving dimethyl fumarate. N Engl J Med 2015;372(15):1474–6.
Gerevini S, Capra R, Bertoli D, Sottini A, Imberti L. Immune profiling of a patient with alemtuzumab-associated progressive multifocal leukoencephalopathy. Mult Scler 2019;25(8):1196–201.
Neff RT, Hurst FP, Falta EM, Bohen EM, Lentine KL, Dharnidharka VR, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation 2008;86:1474–8.
Wathes R, Moule S, Milojkovic D. Progressive multifocal leukoencephalopathy associated with ruxolitinib. N Engl J Med 2013;369(2):197–8.
Carson KR, Focosi D, Major EO, Petrini M, Richey EA, West DP, et al. Monoclonal antibody-associated progressive multifocal leucoencephalopathy in patients treated with rituximab, natalizumab, and efalizumab: a Review from the Research on Adverse Drug Events and Reports (RADAR) Project. [Review] [77 refs]. Lancet Oncol 2009;10(8):816–24.
Spencer CM, Crabtree-Hartman EC, Lehmann-Horn K, Cree BA, Zamvil SS. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurol Neuroimmunol Neuroinflamm 2015;2(3):e76.
Clifford D, Gass A, Richert N, Tornatore C, Vermersch P, Hughs R, et al. Cases reported as progressive multifocal leukoencephalopathy in Ocrelizumab-treated patients with multiple sclerosis. ECTRIMS abstract. 2019.
Hill-Cawthorne GA, Button T, Tuohy O, Jones JL, May K, Somerfield J, et al. Long term lymphocyte reconstitution after alemtuzumab treatment of multiple sclerosis. J Neurol Neurosurg Psychiatry 2012;83(3):298–304.
Mateen FJ, Muralidharan R, Carone M, van de Beek D, Harrison DM, Aksamit AJ, et al. Progressive multifocal leukoencephalopathy in transplant recipients. Ann Neurol 2011;70(2):305–22.
Wykes MN, Lewin SR. Immune checkpoint blockade in infectious disease. Nat Rev Immunol 2018;18(2):91–104.
Tan CS, Bord E, Broge TA, Jr., Glotzbecker B, Mills H, Gheuens S, et al. Increased program cell death-1 expression on T lymphocytes of patients with progressive multifocal leukoencephalopathy. J Acquir Immune Defic Syndr 2012;60(3):244–8.
Hoang E, Bartlett NL, Goyal MS, Schmidt RE, Clifford DB. Progressive multifocal leukoencephalopathy treated with nivolumab. J Neuro-Oncol 2019;25(2):284–7.
Marzocchetti A, Tompkins T, Clifford DB, Gandhi RT, Kesari S, Berger JR, et al. Determinants of survival in progressive multifocal leukoencephalopathy. Neurology 2009;73(19):1551–8.
Neil EC, DeAngelis LM. Progressive multifocal leukoencephalopathy and hematologic malignancies: a single cancer center retrospective review. Blood Adv 2017;1(23):2041–5.
Carson KR, Evens AM, Richey EA, Habermann TM, Focosi D, Seymour JF, et al. Progressive multifocal leukoencephalopathy following rituximab therapy in HIV negative patients: A report of 57 cases from the Research on Adverse Drug Event and Reports (RADAR) project. Blood 2009;113(20):4834–40.
Kupper C, Heinrich J, K. Kamm, Bucklein V, Rothenfusser S, Straube A. Pembrolizumab for progressive multifocal leukoencephalopathy due to primary immunodeficiency. Neurol Neuroimmunol Neuroinflamm. 2019;6(6):e628.
Pawlitzki M, Schneider-Hohendorf T, Rolfes L, Meuth S, Wiendl H, Schwab N, et al. Ineffective treatment of PML with pembrolizumab. Neurol Neuroimmunol Neuroinflamm 2019;6(6):e627.
Medrano C, Vergez F, Mengelle C, Faguer S, Kamar N, Del Bello A. Effectiveness of Immune Checkpoint Inhibitors in Transplant Recipients with Progressive Multifocal Leukoencephalopathy. Emerg Infect Dis 2019;25(11):2145–7.
Pellegrini M, Calzascia T, Toe J, Preston SP, Lin AE, Elford AR, et al. IL-7 Engages Multiple Mechanisms to Overcome Chronic Viral Infection and Limit Organ Pathology. Cell 2011;144(4):601–13.
Parish IA, Kaech SM. IL-7 Knocks the Socs Off Chronic Viral Infection. Cell 2011;144(4):467–8.
Soleimani-Meigooni DN, Schwetye KE, Angeles MR, Ryschkewitsch CF, Major EO, Dang X, et al. JC virus granule cell neuronopathy in the setting of chronic lymphopenia treated with recombinant interleukin-7. J Neuro-Oncol 2017;23(1):141–6.
Miskin DP, Chalkias SG, Dang X, Bord E, Batson S, Koralnik IJ. Interleukin-7 treatment of PML in a patient with idiopathic lymphocytopenia. Neurol Neuroimmunol Neuroinflamm 2016;3(2):e213.
Przepiorka D, Jaeckle KA, Birdwell RR, Fuller GN, Kumar AJ, Huh YO, et al. Successful treatment of progressive multifocal leukoencephalopathy with low-dose interleukin-2. Bone Marrow Transplant 1997;20:983–7.
Re D, Bamborschke S, Feiden W, Schröder R, Lehrke R, Diehl V, et al. Case report: Progressive multifocal leukoencephalopathy after autologous bone marrow transplantation and alpha-interferon immunotherapy. Bone Marrow Transplant 1999;23:295–8.
Kunschner L, Scott TF. Sustained recovery of progressive multifocal leukoencephalopathy after treatment with IL-2. Neurology 2005;65:1510.
Sospedra M, Schippling S, Yousef S, Jelcic I, Bofill-Mas S, Planas R, et al. Treating Progressive Multifocal Leukoencephalopathy With Interleukin 7 and Vaccination With JC Virus Capsid Protein VP1. Clin Infect Dis 2014;59(11):1588–92.
Geoghegan EM, Pastrana DV, Schowalter RM, Ray U, Gao W, Ho M, et al. Infectious Entry and Neutralization of Pathogenic JC Polyomaviruses. Cell Rep 2017;21(5):1169–79.
Wollebo HS, Bellizzi A, Kaminski R, Hu W, White MK, Khalili K. CRISPR/Cas9 System as an Agent fro Eliminating Polyomavirus JC Infection. PLoS ONE. 2015;10(9).
Clifford DB. Neurological immune reconstitution inflammatory response: riding the tide of immune recovery. Curr Opin Neurol 2015;28(3):295–301.
Wattjes MP, Verhoeff L, Zentjens W, Killestein J, van Munster ET, Barkhof F, et al. Punctate lesion pattern suggestive of perivascular inflammation in acute natalizumab-associated progressive multifocal leukoencephalopathy: productive JC virus infection or preclinical PML-IRIS manifestation? J Neurol Neurosurg Psychiatry 2013;84(10):1176–7.
Whiteman ML, Post MJ, Berger JR, Tate LG, Bell MD, Limonte LP. Progressive multifocal leukoencephalopathy in 47 HIV-seropositive patients: neuroimaging with clinical and pathologic correlation. Radiology 1993;187(1):233–40.
Wijburg MT, Witte BI, Vennegoor A, Roosendaal SD, Sanchez E, Liu Y, et al. MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. J Neurol Neurosurg Psychiatry 2016;87(10):1138–45.
Hodel J, Outteryck O, Verclytte S, Deramecourt V, Lacour A, Pruvo JP, et al. Brain Magnetic Susceptibility Changes in Patients with Natalizumab-Associated Progressive Multifocal Leukoencephalopathy. AJNR Am J Neuroradiol 2015;36(12):2296–302.
Yousry TA, Pelletier D, Cadavid D, Gass A, Richert ND, Radue EW, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2012;72(5):779–87.
Tan IL, McArthur JC, Clifford DB, Major EO, Nath A. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 2011;77(11):1061–7.
Wattjes MP, Wijburg MT, Vennegoor A, Witte BI, de Vos M, Richert ND, et al. MRI characteristics of early PML-IRIS after natalizumab treatment in patients with MS. J Neurol Neurosurg Psychiatry 2016;87(8):879–84.
Sanchez P, Meca-Lallana V, J. Vivancos. Tumefactive multiple sclerosis lesions associated with fingolimod treatment: Report of 5 cases. Mult Scler Relat Disord. 2018;25:95–8.
Fine AJ, Sorbello A, Kortepeter C, Scarazzini L. Progressive multifocal leukoencephalopathy after natalizumab discontinuation. Ann Neurol 2014;75(1):108–15.
Martin-Blondel G, Cuzin L, Delobel P, Cuvinciuc V, Dumas H, Alvarez M, et al. Is maraviroc beneficial in paradoxical progressive multifocal leukoencephalopathy-immune reconstitution inflammatory syndrome management? AIDS 2009;23(18):2545–6.
Sierra-Madero JG, Ellenberg SS, Rassool MS, Tierney A, Belaunzaran-Zamudio PF, Lopez-Martinez A, et al. Effect of the CCR5 antagonist maraviroc on the occurrence of immune reconstitution inflammatory syndrome in HIV (CADIRIS): a double-blind, randomised, placebo-controlled trial. Lancet HIV 2014;1(2).
Martin-Blondel G, Bauer J, Uro-Coste E, Biotti D, Averseng-Peaureaux D, Fabre N, et al. Therapeutic use of CCR5 antagonists is supported by strong expression of CCR5 on CD8(+) T cells in progressive multifocal leukoencephalopathy-associated immune reconstitution inflammatory syndrome. Acta Neuropathol 2015;129(3):463–5.
Stork L, Bruck W, Bar-Or A, Metz I. High CCR5 expression in natalizumab-associated progressive multifocal leukoencephalopathy immune reconstitution inflammatory syndrome supports treatment with the CCR5 inhibitor maraviroc. Acta Neuropathol 2015;129(3):467–8.
Scarpazza C, Prosperini L, Mancinelli CR, De Rossi N, Lugaresi A, Capobianco M, et al. Is maraviroc useful in multiple sclerosis patients with natalizumab-related progressive multifocal leukoencephalopathy? J Neurol Sci 2017;378:233–7.
De Luca A, Giancola ML, Ammassari A, Grisetti S, Paglia MG, Gentile M, et al. The Effect of Potent Antiretroviral Therapy and JC Virus Load in Cerebrospinal Fluid on Clinical Outcome of Patients with AIDS-Associated Progressive Multifocal Leukoencephalopathy. J Infect Dis 2000;182(4):1077–83.
Yiannoutsos CT, Major EO, Curfman B, Jensen PN, Gravell M, Hou J, et al. Relation of JC virus DNA in the cerebrospinal fluid to survival in acquired immunodeficiency syndrome patients with biopsy-proven progressive multifocal leukoencephalopathy. AnnNeurol 1999;45(6):816–21.
Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection. Clinical manifestations and treatment with steroids. Neurology 2009;72:1458–64.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM1
(PDF 911 kb)
Rights and permissions
About this article
Cite this article
Dunham, S.R., Schmidt, R. & Clifford, D.B. Treatment of Progressive Multifocal Leukoencephalopathy Using Immune Restoration. Neurotherapeutics 17, 955–965 (2020). https://doi.org/10.1007/s13311-020-00848-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-020-00848-z