Abstract
Purpose
Oncogenic KRAS mutations are found in over 90% of pancreatic ductal adenocarcinomas (PDACs). As yet, however, no effective therapies are available for KRAS-induced malignancies. Therefore, research aimed at the identification of KRAS targets with therapeutic potential is warranted. Our goal was to investigate Aurora A (AURKA) and targeting protein for Xklp2 (TPX2) as potential therapeutic targets in PDAC.
Methods
AURKA and TPX2 expression was assessed using RNAseq and qRT-PCR in PDAC patient samples and matched non-tumor pancreatic tissues. Publicly available PDAC datasets were used to investigate associations of AURKA and TPX2 expression levels with patient survival and the presence of KRAS mutations. Next, we used an Aurora kinase inhibitor, or KRAS, AURKA and TPX2 targeting using RNA interference in KRAS-mutant PDAC cells and, subsequently, analyzed their clonogenic and anchorage-independent growth and migration.
Results
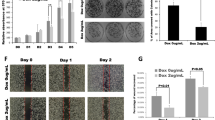
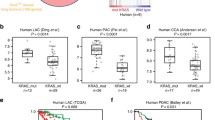
We found that relative to matched non-tumor tissues, PDAC tumors displayed significantly higher expression levels of AURKA and TPX2. In addition, we found that AURKA and TPX2 were co-expressed in PDAC datasets, and that high expression levels of AURKA and TPX2 were associated with a shorter patient survival and with the presence of oncogenic KRAS mutations. In addition, we found that siRNA-mediated KRAS targeting in KRAS-mutant PDAC cells reduced AURKA and TPX2 expression. Furthermore, targeting AURKA or TPX2 in KRAS-mutant PDAC cells reduced their clonogenic and anchorage-independent growth, as well their migration.
Conclusions
From our data we conclude that AURKA and TPX2 may act as KRAS biomarkers in PDAC that can predict a worse prognosis, and that AURKA or TPX2 targeting in PDAC cells may reduce their transformed phenotype. These results indicate that AURKA and TPX2 may serve as promising targets to be explored for KRAS-mutant PDAC therapy.






Similar content being viewed by others
References
D. Zeitouni, Y. Pylayeva-Gupta, C.J. Der, K.L. Bryant, KRAS Mutant pancreatic cancer: No lone path to an effective treatment. Cancers (Basel). 18(8) (2016)
A.G. Stephen, D. Esposito, R.K. Bagni, F. McCormick, Dragging ras back in the ring. Cancer Cell. 25, 272–281 (2014)
P. Lito, A. Saborowski, J. Yue, M. Solomon, E. Joseph, S. Gadal, M. Saborowski, E. Kastenhuber, C. Fellmann, K. Ohara, K. Morikami, T. Miura, C. Lukacs, N. Ishii, S. Lowe, N. Rosen, Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell. 25, 697–710 (2014)
M. Malumbres, I. Pérez de Castro, Aurora kinase A inhibitors: promising agents in antitumoral therapy. Expert. Opin. Ther. Targets. 18, 1377–1393 (2014)
A. Tang, K. Gao, L. Chu, R. Zhang, J. Yang, J. Zheng, Aurora kinases: novel therapy targets in cancers. Oncotarget. 8, 23937–23954 (2017)
D. Li, J. Zhu, P.F. Firozi, J.L. Abbruzzese, D.B. Evans, K. Cleary, H. Friess, S. Sen, Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin. Cancer. Res. 9, 991–997 (2003)
S. Rojanala, H. Han, R.M. Muñoz, W. Browne, R. Nagle, D.D. Von Hoff, D.J. Bearss, The mitotic serine threonine kinase, Aurora-2, is a potential target for drug development in human pancreatic cancer. Mol. Cancer. Ther. 3, 451–457 (2004)
J. Zhu, J.L. Abbruzzese, J. Izzo, W.N. Hittelman, D. Li, AURKA amplification, chromosome instability, and centrosome abnormality in human pancreatic carcinoma cells. Cancer Genet. Cytogenet. 159, 10–17 (2005)
R.J. Bischoff, L. Anderson, Y. Zhu, K. Mossie, L. Ng, B. Souza, B. Schryver, P. Flanangan, F. Clairvoyant, C. Ginther, C.S. Chan, M. Novotny, J.D. Slamon, G.D. Plowman, A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 17, 3050–3065 (1998)
H. Zhou, J. Kuang, L. Zhong, W.L. Kuo, J.W. Gray, A. Sahin, B.R. Brinkley, S. Sen, Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat. Genet. 20, 189–193 (1998)
X. Wang, Y.X. Zhou, W. Qiao, Y. Tominaga, M. Ouchi, T. Ouchi, C.X. Deng, Overexpression of aurora kinase A in mouse mammary epithelium induces genetic instability preceding mammary tumor formation. Oncogene. 16, 7148–7158 (2006)
A. Katsha, A. Belkhiri, L. Goff, W. El-Rifai, Aurora kinase A in gastrointestinal cancers: time to target. Mol. Cancer. 14, 106 (2015)
M. Giuberrini, I.A. Asteriti, J. Scrofani, M. de Luca, C. Lindon, P. Lavia, G. Guarguaglini, Control of Aurora-A stability through interaction with TPX2. J. Cell. Sci. 124, 113–122 (2011)
A. Zorba, V. Buosi, S. Kutter, N. Kern, F. Pontiggia, Y.J. Cho, J. Kernl, Molecular mechanism of Aurora A kinase autophosphorylation and its allosteric activation by TPX2. eLife. 3, e02667 (2014)
I.A. Asteriti, W.M. Rensen, C. Lindon, P. Laiva, G. Guarguaglini, The Aurora-A/TPX2 complex: A novel oncogenic holoenzyme? Biochim. Biophys. Acta. 1806, 230–239 (2010)
G. Neumayer, C. Belzil, O.J. Gruss, M.D. Nguyen, TPX2: of spindle assembly, DNA damage response, and cancer. Cell Mol. Life Sci. 71, 3027–3047 (2014)
S.E. Morgan-Lappe, L.A. Tucker, X. Huang, Q. Zhang, A.V. Sarthy, D. Zakula, L. Vernetti, M. Schurdark, J. Wang, S.W. Fesik, Identification of Ras-Related Nuclear Protein, Targeting Protein for Xenopus Kinesin-like Protein 2, and Stearoyl-CoA Desaturase 1 as promising cancer targets from an RNAi-based screen. Cancer Res. 67, 4390–4398 (2007)
S.L. Warner, B.J. Stephens, S. Nwokenkwo, G. Hostetter, A. Sugeng, M. Hidalgo, J.M. Trent, H. Han, D.D. Von-Hoff, Validation of TPX2 as a potential therapeutic target in pancreatic cancer cells. Clin. Cancer Res. 1, 6519–6528 (2009)
Y. Huang, W. Guo, H. Kan, TPX2 is a prognostic marker and contributes to growth and metastasis of human hepatocellular carcinoma. Int. J. Mol. Sci. 15, 18148–18161 (2014)
B. Liang, C. Jia, Y. Huang, H. He, J. Li, H. Liao, X. Liu, X. Liu, X. Bai, D. Yang, TPX2 level correlates with hepatocellular carcinoma cell proliferation, apoptosis, and EMT. Dig. Dis. Sci. 60, 2360–2372 (2015)
J.J. Gu, J.H. Zhang, H.J. Chen, S.S. Wang, TPX2 promotes glioma cell proliferation and invasion via activation of the AKT signaling pathway. Oncol. Lett. 12, 5015–5022 (2016)
A.H. Sillars-Hardebol, B. Carvalho, M. Tijssen, J.A. Beliën, M. de Wit, P.M. Delis-van-Diemen, F. Potén, M.A. van de Wiel, R.J. Fijneman, G.A. Meijer, TPX2 and AURKA promote 20q amplicon-driven colorectal adenoma to carcinoma progression. Gut. 61, 1568–1575 (2012)
G. Garrido, I. Vernos, Non-centrosomal TPX2-dependent regulation of the Aurora A kinase: Functional implications for healthy and pathological cell division. Front. Oncol. 15, 88 (2016)
M. Tatsuka, S. Sato, S. Kitajima, S. Suto, H. Kawai, M. Miayauchi, I. Ogawa, M. Meada, T. Ota, T. Takata, Overexpression of Aurora-A potentiates HRAS-mediated oncogenic transformation and is implicated in oral carcinogenesis. Oncogene. 24, 1122–1127 (2005)
Y.S. Tseng, J.C. Lee, C.Y. Huang, H.S. Liu, Aurora-A overexpression enhances cell-aggregation of Ha-ras transformants through the MEK/ERK signaling pathway. BMC Cancer. 12, 435 (2009)
G. Yang, I. Mercado-Uribe, A.S. Multani, S. Sen, I.M. Shih, K.K. Wong, D.M. Gershenson, J. Liu, RAS promotes tumorigenesis through genomic instability induced by imbalanced expression of Aurora-A and BRCA2 in midbody during cytokinesis. Int. J. Cancer. 133, 275–285 (2013)
E.O. dos Santos, T.C. Carneiro-Lobo, M.N. Aoki, E. Levantini, D.S. Bassères, Aurora kinase targeting in lung cancer reduces KRAS-induced transformation. Mol. Cancer. 15, 12 (2016)
M.E. Ritchie, B. Phipson, D. Wu, Y. Hu, C.W. Law, W. Shi, G.K. Smyth, Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015)
T. Therneau, A Package for Survival Analysis in S. version 2.38. (R CRAN, 2015) https://CRAN.R-project.org/package=survival Accessed 20 Nov 2018
S. Carter, A.C. Eklund, I.S. Kohane, L.N. Harris, Z. Szallasi, A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat. Genet. 38, 1043–1048 (2006)
R. Aguirre-Gamboa, H. Gomez-Rueda, A. Martínez-Ledesma, R. Chacolla-Huaringa, A. Rodriguez-Barrientos, J.G. Tamez-Peña, V. Treviño, SurvExpress: An online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoSOne. 9, e74250 (2013)
J.K. Stratford, D.J. Bentrem, J.M. Anderson, C. Fan, K.A. Volmar, J.S. Marron, E.D. Routh, L.S. Caskey, J.C. Samuel, C.J. Der, L.B. Thorne, B.F. Calvo, H.J. Kim, M.S. Talamonti, C.A. Iacobuzio-Donahue, M.A. Hollingsworth, C.M. Perou, J.J. Yeh, A six-gene signature predicts survival of patients with localized pancreatic ductal adenocarcinoma. PLoS Med. 13, 7 (2010)
P.C. Brooks, S. Strömblad, L.C. Sanders, T.L. von Schalscha, R.T. Aimes, W.G. Stetler-Stevenson, J.P. Quigley, D.A. Cheresh, Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 85, 683–693 (1996)
M. Choi, H. Bien, A. Mofunanya, S. Powers, Challenges in Ras therapeutics in pancreatic cancer. Semin. Cancer Biol. 54, 101–108 (2019)
M.R. Janes, J. Zhang, L.S. Li, R. Hansen, U. Peters, X. Guo, Y. Chen, A. Babbar, S.J. Furdaus, L. Darjania, J. Feng, J.H. Chen, S. Li, Y.O. Long, C. Thach, Y. Liu, A. Zarieh, T. Ely, J.M. Kucharski, L.V. Kessler, T. Wu, K. Yu, Y. Wang, Y. Yao, X. Deng, P.P. Zarrinkar, D. Brehmer, D. Dhanak, M.V. Lorenzi, D. Hu-Lowe, M.P. Patricelli, P. Ren, Y. Liu, Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell. 172, 578–589 (2018)
J. Tayou, Identification of subsets of actionable genetic alterations in KRAS-mutant lung cancers using association rule mining. Cell Oncol. 41, 395–408 (2018)
H. Katayama, K. Sasai, H. Kawai, Z.M. Yuan, J. Bondaruk, F. Suzuki, S. Fujii, R.B. Arlinghaus, B.A. Czerniak, S. Sen, Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat. Genet. 36, 55–62 (2004)
G. Pascreau, F. Eckerdt, A.L. Lewellyn, C. Prigent, J.L. Maller, Phosphorylation of p53 is regulated by TPX2-Aurora A in xenopus oocytes. J. Biol. Chem. 284, 5497–5505 (2009)
Y. Takahashi, P. Sheridan, A. Niida, G. Sawada, R. Uchi, H. Mizuno, J. Kurashiege, K. Sugimachi, S. Sasaki, Y. Shimada, K. Hase, M. Kusunoki, S. Kudo, M. Watanabe, K. Yamada, K. Sugihara, H. Yamamoto, A. Suzuki, Y. Doki, S. Miyno, M. Mori, K. Mimori, The AURKA/TPX2 axis drives colon tumorigenesis cooperatively with MYC. Ann. Oncol. 26, 935–942 (2015)
A. Gupta, R. Jain, D. Wahi, S. Goyal, S. Jamal, A. Grover, Abrogation of AuroraA-TPX2 by novel natural inhibitors: molecular dynamics-based mechanistic analysis. J. Recept. Signal Transduct. Res. 35, 626–633 (2015)
M. Janecek, M. Rossman, P. Sharma, A. Emery, D.J. Huggins, S.R. Stockwell, J.E. Stokes, Y.S. Tan, E.G. Almeida, B. Hardwick, A.J. Narvaez, M. Hyvönen, D.R. Spring, G.J. McKenzie, A.R. Venkitaraman, Allosteric modulation of AURKA kinase activity by a small-molecule inhibitor of its protein-protein interaction with TPX2. Sci. Rep. 6, 28528 (2016)
M.A. Schneider, P. Christopoulos, T. Muley, A. Warth, U. Klingmueller, M. Thomas, F.J. Herth, H. Dienemann, N.S. Mueller, F. Theis, M. Meister, AURKA, DLGAP5, TPX2, KIF11 and CKAP5: Five specific mitosis-associated genes correlate with poor prognosis for non-small cell lung cancer patients. Int. J. Oncol. 50, 365–372 (2017)
P. Mohan, J. Castellsague, J. Jiang, K. Allen, H. Chen, O. Nemirovsky, M. Spyra, K. Hu, L. Kluwe, M.A. Pujana, A. Villanueva, V.F Mautner, J.J. Keats, S.E. Dunn, C. Lazaro, C.A. Maxwell, Genomic imbalance of HMMR/RHAMM regulates the sensitivity and response of malignant peripheral nerve sheath tumour cells to Aurora Kinase inhibition. Oncotarget. 4, 80-93 (2013)
A. Chowdhury, S. Chowdhury, M.Y. Tsai, A novel Aurora kinase A inhibitor MK-8745 predicts TPX2 as a therapeutic biomarker in non-Hodgkin lymphoma cell lines. Leuk. Lymphoma. 53, 462–471 (2011)
A.B. D’Assoro, T. Haddad, E. Galanis, Aurora-A kinase as a promising therapeutic target in cancer. Front. Oncol. 5, 295 (2016)
I.P. Gladhaug, A. Westgaard, A.R. Schjølberg, E. Burum-Auesen, E. Pomianowska, O.P. Clausen, Spindle proteins in resected pancreatic head adenocarcinomas: BubR1 is an independent prognostic factor in pancreatobiliary-type tumours. Histopathology. 56, 345–355 (2010)
A. Vallejo, N. Perurena, E. Guruceaga, P.K. Mazur, S. Martinez-Canarias, C. Zandueta, K. Valencia, A. Arricibita, D. Gwinn, L.C. Sayles, C.H. Chuang, L. Guembe, P. Bailey, D.K. Chang, A. Biankin, M. Ponz-Sarvise, J.B. Andersen, P. Khatri, A. Bozec, E.A. Sweet-Cordero, J. Sage, F. Lecanda, S. Vicent, An integrative approach unveils FOSL1 as an oncogene vulnerability in KRAS-driven lung and pancreatic cancer. Nat. Commun. 8, 14294 (2017)
Y. Xie, S. Zhu, M. Zhong, M. Yang, X. Sun, J. Liu, G. Kroemer, M. Lotze, H.J. Zeh 3rd, R. Kang, D. Tang, Inhibition of Aurora Kinase A induces necroptosis in pancreatic carcinoma. Gastroenterology. 153, 1429–1443 (2017)
T. Kobayash, K. Nakazono, M. Tokuda, Y. Mashima, B.D. Dynlacht, H. Itoh, HDAC2 promotes loss of primary cilia in pancreatic ductal adenocarcinoma. EMBO Rep. 18, 334–343 (2017)
M. Umstead, J. Xiong, Q. Qi, Y. Du, H. Fu, Aurora kinase A interacts with H-Ras and potentiates Ras-MAPK signaling. Oncotarget 8, 28359–28372 (2017)
A.T. Boutin, W.T. Liao, M. Wang, S.S. Hwang, T.V. Karpinets, H. Cheung, G.C. Chu, S. Jiang, J. Hu, K. Chang, E. Vilar, X. Song, J. Zhang, S. Kopetz, A. Futreal, Y.A. Wang, L.N. Kwong, R.A. DePinho, Oncogenic Kras drives invasion and maintains metastases in colorectal cancer. Genes Dev. 31, 370–382 (2017)
J. Wang, K. Nikhil, K. Viccaro, L. Chang, M. Jacobsen, G. Sandusky, K. Shah, The Aurora-A-Twist1 axis promotes highly aggressive phenotypes in pancreatic carcinoma. J. Cell Sci. 130, 1078–1093 (2017)
Y. Zhao, X. Hu, L. Wei, D. Song, J. Wang, L. You, H. Saiyin, Z. Li, W. Yu, L. Yu, J. Ding, J. Wu, PARP10 suppresses tumor metastasis through regulation of Aurora A activity. Oncogene. 37, 2921–2935 (2018)
D. Huang, J. Chen, C. Yang, M. Wang, TPX2 silencing mediated by joint action of microvesicles and ultrasonic radiation inhibits the migration and invasion of SKOV3 cells. Mol. Med. Rep. 17, 7627–7635 (2018)
B. Liang, W. Zheng, L. Fang, L. Wu, F. Zhou, X. Yin, X. Yu, Z. Zou, Overexpressed targeting protein for Xklp2 (TPX2) serves as a promising prognostic marker and therapeutic target for gastric cancer. Cancer Biol. Ther. 17, 824–832 (2016)
X. Wang, N. Lu, B. Niu, X. Chen, J. Xie, N. Cheng, Overexpression of Aurora-A enhances invasion and matrix metalloproteinase-2 expression in esophageal squamous cell carcinoma cells. Mol. Cancer Res. 10, 588–596 (2012)
Acknowledgements
This work was supported by a Young Investigator Grant (2010/52685-9) and a Research Grant (2016/19757-2) from the Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP) to D.S.B., an Established Researcher fellowship (308663/2018-7) to E.M.R. by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), CNPq Ph.D. fellowships to E.O.S. (166803/2013-8) and L.C.S. (155381/2016-4) and by an undergraduate CNPq fellowship to V.H (PIBIC 2017/701). This work was also supported by a Ph.D. fellowhip to S.M.G-F. by the graduate program in Biochemistry and Molecular Biology of the University of São Paulo, which is sponsored by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, PROEX 1888/2016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
The study involving human samples was approved by the Ethics Committee of the AC Camargo Cancer Center (CAAE: 15059213.0.0000.5432). All procedures used were in accordance with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gomes-Filho, S.M., dos Santos, E.O., Bertoldi, E.R.M. et al. Aurora A kinase and its activator TPX2 are potential therapeutic targets in KRAS-induced pancreatic cancer. Cell Oncol. 43, 445–460 (2020). https://doi.org/10.1007/s13402-020-00498-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00498-5