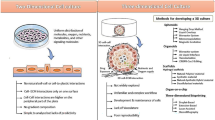
Abstract
Large animal models have been widely used to facilitate the translation of mesenchymal stem cells (MSC) from the laboratory to patient. MSC, with their multi-potent capacity, have been proposed to have therapeutic benefits in a number of pathological conditions. Laboratory studies allow the investigation of cellular and molecular interactions, while small animal models allow initial ‘proof of concept’ experiments. Large animals (dogs, pigs, sheep, goats and horses) are more similar physiologically and structurally to man. These models have allowed clinically relevant assessments of safety, efficacy and dosing of different MSC sources prior to clinical trials. In this review, we recapitulate the use of large animal models to facilitate the use of MSC to treat myocardial infarction—an example of one large animal model being considered the ‘gold standard’ for research and osteoarthritis—an example of the complexities of using different large animal models in a multifactorial disease. These examples show how large animals can provide a research platform that can be used to evaluate the value of cell-based therapies and facilitate the process of ‘bench to bedside’.
Similar content being viewed by others
Introduction
Animals are used in research where there is a need to study the effect of a treatment on a whole tissue or living organism (Barré-Sinoussi and Montagutelli 2015). Humans and animals share many similarities both morphologically and pathologically and animas are regularly used to study disease onset, progression and treatment (Solinas et al. 2014). In the development of novel therapeutics, animal models can also provide vital information on safety and efficacy prior to human studies (Bianco et al. 2013). All animal research is tightly regulated by the country in which it is being undertaken and research on animals within the EU is regulated under Directive 2010/63/EU (Macrì et al. 2013). This directive was established in all EU states in 2013 to ensure a harmoniously high standard of animal research (Macrì et al. 2013). The directive ensures a contentious effort to implement strategies to reduce the number of animals used in research while refining techniques to reduce predicted pain, suffering, distress and/or lasting pain whilst also improving animal husbandry. Animal experiments are conducted on a wide variety of species including invertebrates, fish, birds and mammals (with mammalian species being divided into ‘small’ animal or ‘large’ animal models).
An animal is considered a ‘large animal’ when the species in question is non rodent, rabbit or guinea pig (Thomas et al. 2012). The more commonly used large animal models in research include horses, cows, pigs, sheep, goats, primates and dogs, and the choice of animal model depends on multiple factors, including the type of experiment, its duration, husbandry costs, handling logistics and measurement parameters (Kuyinu et al. 2016).
Whilst small animals have been invaluable in furthering modern understanding of disease by providing an opportunity to conduct research cheaply, rapidly and with a degree of complexity not offered by in vitro experiments or other species, in some situations the information that can be provided by large animals is required to answer specific research questions (Moran et al. 2016; Ziegler et al. 2016). Large animal models offer advantages over small animal models in many areas. They are more similar physiologically and anatomically to man (size, tissue structure and life span) and large animals are an ‘out bred’ population that more closely represents the heterogeneity of the human population than the ‘inbred’ small animal strains used in research (Salvatore et al. 2008). Large animals are phylogenetically closer to humans than rodents and therefore, at a molecular level, they have greater sequence homology with humans making interpretation of molecular events in large animals more relevant to man (Henze and Urban 2010). Practically, the consequence of working with a large animal means that more body fluids and cells can be collected with which to perform experiments.
To illustrate how using large animals have facilitated the process of moving MSC from ‘bench to bedside’, two examples will be considered in this review—the treatment of myocardial infarction (MI) and osteoarthritis (OA). The former represents an example of one single large animal model being considered the ‘gold standard’ for research, while the latter is an example of the complexities of using large animal models in a multifactorial disease.
Large animals models for treating myocardial disease using MSC
There has been a recent increase in the incidence of MI worldwide (Rumana et al. 2008). This is due to many factors such as an ageing population, more sedentary lifestyles and generally poorer diets (Mohseni et al. 2017). MI is diagnosed as a cessation of correct blood flow to the heart, leading, in clinical practice, to sudden death, or ischaemia and subsequent loss of cardiomyocytes (Chiong et al. 2011; Reddy 2015). The chances of surviving one MI are high, but post MI complications are of clinical significance (Chiong et al. 2011). Localised myocardium loss leads to heart wall thinning and ventricle dysfunction (Lu et al. 2015). In order to maintain heart function, the left ventricle dilates to maintain stroke volume and cardiac output (Mohseni et al. 2017). However, left ventricular dilatation leads to heart failure and eventual death and MI clearly represents a key pathology that requires therapy (Reddy 2015). Over the past 40 years, our understanding of MI has increased and, with this, so have the number of MI related publications (Saleh and Ambrose 2018).
The possibility of using MSC to regenerate cardiomyocytes became possible when it was demonstrated in vitro that, in addition to the well-recognised differentiation products of MSC (into osteoblasts, adipocytes and chondrocytes), MSC can be differentiated into cardiac cell types (White et al. 2016; Szaraz et al. 2017; Guo et al. 2018). For example, Szaraz et al. (2017) differentiated human umbilical MSC into ‘cardiac like cells’ that expressed cardiac myocyte differentiation markers such as myocyte enhanced factor 2C, cardiac troponin T, heavy chain cardiac myosin, signal regulatory protein α and connexion 43. Similarly, Markmee et al. (2017) showed that after 21 days in cardiogenic culture medium, MSC displayed the cardiomyocyte markers GATA binding protein 4, cardiac muscle troponin, connexin 43 and Nkx2.5. Cross-talk between MSC and cardiomyocytes was demonstrated by Gao et al. (2016) who showed that co-culture of MSC with neonatal rat ventricular myocytes lead to the development of partial electrical properties similar to the cardiomyocytes (Gao et al. 2016).
In addition to the ability of MSC to differentiate into ‘cardiac-like cells’, it has also been shown that MSC can support cardiac cell viability via secreted factors. Ismail et al. (2014) created a model of hydrogen peroxide-induced cardiomyocyte injury and showed that neonatal cardiomyocytes and the cardiac myoblast cell line H9c2 both had significantly increased viability and reduced apoptosis in the presence of MSC secreted SC1 (Ismail et al. 2014). Xiang et al. (2009) also showed that the application of MSC conditioned media to neonatal rat cardiomyocytes and reduced cardiomyocyte apoptosis via effects on the mitochondrial pathway (Xiang et al. 2009).
Following these encouraging in vitro results, subsequent small animal studies showed that MSC had therapeutic efficacy in a MI model. Functionally, MSC were shown to have a number of positive effects including improving left ventricle function, increasing vascular density, decreasing scar size (López et al. 2013; Wang et al. 2018), left ventricle stroke volumes and ejection fractions (Dai et al. 2005) and increasing remodelling of gap junctions (Dai et al. 2005; López et al. 2013; Wang et al. 2018). There is also some evidence that MSC differentiate, in situ, into cardiac cells at sites of damage (Nagaya et al. 2005).
However, whilst small animal studies have been useful to show proof of concept for the use of MSC to treat MI, it has been necessary to use large animal models, specifically the porcine ischaemic MI model, to confirm the suitability of this cell therapy in man. Small animal cardiac parameters such as heart rate, coronary architecture and capillary density (Harding et al. 2013) are markedly different to man, whereas large animal hearts are more similar (Harding et al. 2013). The porcine model is the most used for MI research due to the similarities in heart size and coronary anatomy between pigs and humans (Swindle et al. 2012). Also, again on a practical note, the relatively high sequence homology between porcine and human proteins more readily facilitates research enabling commercially purchased reagents to be used (Dreher et al. 2011).
The ‘gold standard’ model of porcine MI that is used in all published papers is the artery occlusion model, in which, a dilation catheter is inflated in the coronary artery. This catheter blocks blood flow to part of the heart causing infarction development. However, the remainder of the heart will continue to receive normal blood perfusion and thus provides a defined border zone between normal and damaged tissue for comparative evaluation (McCall et al. 2012). Schuleri et al. (2009) showed a positive effect of using autologous BM-MSC, administered 12 weeks post infarct to treat MI. Magnet resonance imaging (MRI) was used to assess infarct size, myocardial blood flow and left ventricle function. In this study, an apparent dose-dependent effect of MSC administration on infarct size was observed.
Whilst Schuleri et al. (2009) used autologous MSC in their experimental work, there is much interest in allogeneic MSC therapy. Allogeneic MSC offer significant advantages over autologous MSC including their ease of use, reduced cost and absence of donor site complications (Schuleri et al. 2009). Quevedo et al. (2009) showed that allogeneic MSC are able to regenerate an experimentally created, chronically infarcted myocardium via long-term engraftment (Quevedo et al. 2009). Following MRI, cell fate was confirmed using Y chromosome cell tracking. In comparison to the control group, infarct size reduced by 5.4%, ejection fraction increased by 6.3% and levels of MSC engraftment correlated with functional recovery levels (measured by assessing contractility and myocardial blood flow). In this study, the implanted MSC were only detected within the infarct area or the infarct border with 14% showing evidence of myocyte commitment (assessed by the presence of cardiac transcription factors GATA-4 and Nkx2.5 or structural cardiac proteins α-sarcomeric actin and tropomyosin) (Quevedo et al. 2009). Similarly Williams et al. (2013) also investigated the use of allogeneic MSC with excellent results—a 19.62% reduction in scar size after 12 weeks, progressing to 28.09% after 24 weeks and a functional improvement in heart function (Williams et al. 2013).
The studies reported above all showed positive effect of administrating MSC as early as 12 weeks post infarct creation. However, administration at earlier time points has also been shown to be efficacious, for example, administration at 3 days post infarct (Hatzistergos et al., 2010), suggesting that the optimal time window for therapeutic intervention is not fully established. Lee et al. showed that administering EVs after 30 min post infarct had no effect, thus work continues in the porcine model to determine these important criteria. Examples of these studies are summarised in Table 1.
Due to positive results in the porcine MI model, MSC are now being used in clinical trials to treat a variety of cardiac diseases in man (Table 2). In these clinical trials to date, all have reported that the use of MSC is safe and a significant majority of studies have reported a positive outcome despite a high number of variables in the studies. However, it should be noted that many knowledge gaps still exist and study designs should now attempt to gain knowledge, such as the optimum dosage, cell source and time of injection.
Large animal models for osteoarthritis
In contrast to the single porcine large animal model that has been used to show the efficacy of MSC in the treatment of MI, a variety of large animal models have been used to demonstrate the therapeutic benefits of MSC in the treatment of Osteoarthritis (OA) prior to clinical trials.
OA is the gradual degeneration of articular cartilage within synovial joints (Sharma et al. 2013). It is estimated that, worldwide, eight million people over the age of 65 suffer with this disease (Neogi 2013). OA is the result of structural and functional failures within the synovial joint (Nuki 1999). This is due to the pathological loss of articular cartilage coupled with sub-chondral bone thickening, osteophyte development, ligament degeneration and varying levels of inflammation (Chen et al. 2017). These pathologies all contribute to pain-induced joint morbidity (Chen et al. 2017). OA can be classified into primary and secondary forms based on aetiology. Primary forms of the disease are age-related, whilst trauma is the most common form of secondary OA (Samson et al. 2007).
There are currently no disease-modifying therapeutics licensed for use in OA and there is a huge clinical need for effective therapies. In recent years, MSC have been used to treat OA in pre-clinical and clinical studies. The rationale behind the use of MSC to treat OA was initially proposed to be harnessing the potential of MSC to differentiated into mesodermal tissues including cartilage. It was proposed that MSC, injected into damaged joints, differentiate into the tissues of the joints and healed the lesions. However, more mature understanding of the mechanism of action of MSC suggest that rather than acting as building blocks, they are acting in a paracrine fashion to modulate cellular responses (Kong et al. 2017).
As outlined for MI research above, the pathway to human clinical trials for using MSC as an OA therapeutic is based on in vitro, small animal and then large animal models.
Evidence that MSC have a beneficial effect on the native cells within the joint has been shown in numerous studies (reviewed by (Li et al. 2019). For example, the co-culture of chondrocytes and MSC has been shown to increase glycosaminoglycan synthetic activity as well as increased expression of chondrogenesis-related genes (type II collagen and SOX-9) whilst simultaneously downregulating the expression of osteogenic markers and chondrocyte hypertrophic markers (Bian et al. 2011; Huang et al. 2018; Kim et al. 2018). Similarly, it has been shown that MSC can promote both macroscopic and microscopic healing of meniscal defects, usually in the presence of biocompatible scaffolds (Pabbruwe et al. 2010; Zellner et al. 2010; Mandal et al. 2011; Nerurkar et al. 2011).
In small animals, MSC have been shown to have disease-modifying properties in a number of experimental small OA models, such as in mouse and rabbit anterior cruciate ligament transection models (Chiang et al. 2016). Similarly, Tang et al. (2017) also showed that MSC decreased osteophyte and fibrous tissue formation and increased type II collagen and aggrecan in a rat medial menisectomy model after the administration of MSC (Tang et al. 2017). Improved cartilage repair has also been shown in chemically induced murine arthritis models and in focal cartilage defect models (Kehoe et al. 2014; Mak et al. 2016).
Whilst MSC have been used in small animal OA models as described above, large animals offer significant advantages over small animals for the assessment of the therapeutic benefits of MSC prior to clinical trials. Large animals have similar bone development to man compared to small animals, i.e. they have closed growth plates at skeletal maturity and large animal models of OA occur more slowly than in small animal models, mimicking the natural disease in man (McGovern et al. 2018). However, it must be noted that whilst all large animals will develop OA naturally as they age, there are no models of spontaneous early onset OA as there are in small animals (Bendele et al. 1989; Jimenez et al. 1997; Poole et al. 2010).
Unlike the use of a single ‘gold standard’ large animal model for evaluating the effects of MSC in MI, many models exist for the generation of OA in large animals. Experimental models of large animal OA are primarily surgically induced damage, although there are two reports of the use of MSC to treat chemically induced arthritis. Mokbel et al. (2011) used amphotericin-B in a donkey OA induction model and demonstrated that the injected cells had integrated within the existing cartilage and the reparative effects of the MSC were observed both clinically and radiographically (Mokbel et al. 2011). Barrachina et al. (2018) described the use of bone marrow MSC to treat amphotericin-B induced arthritis in an equine radio-carpal joint. In this study, the application of MSC decreased synovial inflammation, enhanced the gross appearance of the cartilage and delayed proteoglycan loss in comparison to the control. This study also reported differences in outcome between naïve MSC and MSC primed with tumour necrosis factor—alpha (TNFα) and interferon-gamma (IFN-γ). This data is particularly useful in considering the clinical translation of MSC as there is ongoing discussion as to the need for MSC priming/conditioning prior to use (Succar et al. 2016; Barrachina et al. 2018)
Whilst there are only currently two reported studies on the use of MSC to treat chemically induced arthritis in large animal models, many studies have reported the use of different MSC to treat surgically induced arthritis as a proxy for the human disease. (Table 3). In these studies a wide range of large animal species and different surgical techniques have been used to model OA. These techniques include anterior cruciate ligament transection (ACLT), meniscectomy and medial meniscal transection and osteochondral fragment defect models. These are all well-standardised procedures, with each model posing its own advantages and disadvantages (reviewed in (Kuyinu et al. 2016).
Whilst many studies use autologous cells, as discussed previously in the treatment of MI, the use of allogeneic MSC to treat OA is of considerable interest. For example, human BM-MSC were used to treat ACLT induced OA in a porcine model 16 weeks post-surgery (Tseng et al. 2018). At 5 months post implantation, there was a significant difference between the regeneration of new tissue, with the treated group showing evidence of cartilage-like tissue. Similarly, Hatsushika et al.(2014) investigated the effect of allogeneic synovial MSC following partial meniscectomy in a porcine model and showed increased meniscus regeneration and prevention of OA progression by week 16 post-surgery (Hatsushika et al. 2014). Murphy et al. (2003) has also shown that the administration of allogeneic bone marrow MSC following ACTL in goats led to significantly increased tissue regeneration including the meniscus and decreased articular cartilage degeneration, osteophyte remodelling and subchondral sclerosis in comparison to the hyaluronan control(Murphy et al. 2003). These studies are important for the potential clinical applications of MSC as they may suggest there is no requirement for donor matching when using MSC therapeutically.
Whilst the studies above and those reported and summarised in Table 3 shows that MSC had a positive effect in a number of different models of OA, large animal studies have shown that MSC therapies are not always successful. Evaluation of the effects of allogeneic MSC on the development of OA following complete meniscectomy in a sheep model has been reported (Song et al. 2014; Delling et al. 2015). After 12 weeks, MRI, radiography and post-mortem evaluation showed no significant difference in the degree of OA between the treatment group and the control. Similarly, the use of MSC in the osteochondral fragment model of OA induction in horses showed no significant effects (Frisbie et al. 2009). This reporting of negative results from a large animal model is important data, inducing caution in the use of these cells. MSC therapy has widely been touted as a miraculous ‘cure all’, particularly in the popular press and amongst less scrupulous clinicians, and stringent efforts must continue to be made to ensure tight but feasible regulation of these therapies to ensure patient safety, as the use of MSC to treat patients is well underway (Table 4) (Bianco et al. 2013). A number of controlled clinical trials have been reported, with good outcomes in both visual analogue scale for chronic pain and western Ontario and McMaster Universities arthritis index scores (measures of joint morbidity), as well as range of movement, improved pain and joint motility scores following treatment (Lamo-Espinosa et al. 2016; Pers et al. 2016). These studies demonstrate the translation of MSC therapy into man whilst large animal therapeutic trials remain ongoing.
Conclusions
Large animal models have been widely used to facilitate the translation of MSC from the laboratory to patient. The aim of this review is to illustrate how MSC have been translated to man through large animal models. For this, two very different examples have been used—MI (where one gold standard large animal model has been used in one species to show efficacy) and OA (where multiple species and models have been used). It is clear that using multiple models and different experimental approaches makes interpretation of results difficult and the use of a single large animal model is preferable. It is also clear that the majority of publications only report positive outcomes of MSC therapy and that encouragement of the publication of negative outcomes should be made as this will allow a more accurate assessment of therapeutic efficiency. However, used appropriately, large animal models allow clinically relevant assessments of safety, efficacy and dosing prior to clinical trials and continue to provide a research platform that can be used to evaluate the value of cell-based therapies.
References
Al-Najar M, et al. Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J Orthop Surg Res. BioMed central ltd. 2017;12(1). https://doi.org/10.1186/s13018-017-0689-6.
Ascheim DD, Gelijns AC, Goldstein D, Moye LA, Smedira N, Lee S, et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation. Lippincott Williams and Wilkins. 2014;129(22):2287–96. https://doi.org/10.1161/CIRCULATIONAHA.113.007412.
Barrachina L, et al. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Vet Res. 2018;14. https://doi.org/10.1186/s12917-018-1556-3.
Barré-Sinoussi F, Montagutelli X. Animal models are essential to biological research: issues and perspectives. Future Science OA (FSOA). 2015;1(4):63. https://doi.org/10.4155/fso.15.63.
Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circ Res. Lippincott Williams and Wilkins. 2017;121(10):1192–204. https://doi.org/10.1161/CIRCRESAHA.117.310712.
Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (cardiopoietic stem cell therapy in heart failure) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61(23):2329–38. https://doi.org/10.1016/j.jacc.2013.02.071.
Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J Oxford University Press. 2017;38(9):648–60. https://doi.org/10.1093/eurheartj/ehw543.
Bendele AM, White SL, Hulman JF. Osteoarthrosis in Guinea pigs: histopathologic and scanning electron microscopic features. Lab Anim Sci. 1989;39(2):115–21.
Bian L, et al. Coculture of human mesenchymal stem cells and articular chondrocytes reduces hypertrophy and enhances functional properties of engineered cartilage. Tissue Eng Part A. Mary Ann Liebert, Inc. 2011;17(7–8):1137–45. https://doi.org/10.1089/ten.TEA.2010.0531.
Bianco P, Barker R, Brüstle O, Cattaneo E, Clevers H, Daley GQ, et al. Regulation of stem cell therapies under attack in Europe: for whom the bell tolls. European Molecular Biology Organisation (EMBO). 2013;32(11):1489–95. https://doi.org/10.1038/emboj.2013.114.
Butler J, et al. Intravenous allogeneic Mesenchymal stem cells for nonischemic cardiomyopathy: safety and efficacy results of a phase II-A randomized trial. Circ Res. Lippincott Williams and Wilkins. 2017;120(2):332–40. https://doi.org/10.1161/CIRCRESAHA.116.309717.
Chahal J, Gómez-Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, et al. Bone marrow Mesenchymal stromal cell treatment in patients with osteoarthritis results in overall improvement in pain and symptoms and reduces synovial inflammation. Stem Cells Transl Med. John Wiley and Sons Ltd. 2019;8(8):746–57. https://doi.org/10.1002/sctm.18-0183.
Chen D, et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Resh. Nature Publishing Group. 2017;5:16044. https://doi.org/10.1038/boneres.2016.44.
Chen S, Liu Z, Tian N, Zhang J, Yei F, Duan B, et al. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18(11):552–6.
Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94(1):92–5. https://doi.org/10.1016/j.amjcard.2004.03.034.
Chiang E-R, et al. Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PloS One. Public Library of Science. 2016;11(2):e0149835. https://doi.org/10.1371/journal.pone.0149835.
Chiong M, Wang ZV, Pedrozo Z, Cao DJ, Troncoso R, Ibacache M, et al. Cardiomyocyte death: mechanisms and translational implications. Cell Death Dis. Nat Publ Group. 2011;2(12):e244–e244. https://doi.org/10.1038/cddis.2011.130.
Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, et al. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112(2):214–23. https://doi.org/10.1161/CIRCULATIONAHA.104.527937.
Delling U, et al. Longitudinal evaluation of effects of intra-articular mesenchymal stromal cell administration for the treatment of osteoarthritis in an ovine model. Cell Transplant. 2015;24(11):2391–407. https://doi.org/10.3727/096368915X686193.
Dreher F, Kamburov A, Herwig R. Construction of a pig physical interactome using sequence homology and a comprehensive reference human interactome. Evol Bioinforma. Libertas Academica Ltd. 2011;2011(7):119–26. https://doi.org/10.4137/EBO.S8552.
Emadedin, M. et al. (2015) Long-term follow-up of intra-articular injection of autologous mesenchymal stem cells in patients with knee, ankle, or hip osteoarthritis, Arch Iran Med. Academy of Medical Sciences of I.R. Iran, 18(6), pp. 336–344. doi: 015186/AIM.003.
Florea V, et al. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the TRIDENT study). Circ Res. Lippincott Williams and Wilkins. 2017;121(11):1279–90. https://doi.org/10.1161/CIRCRESAHA.117.311827.
Frisbie DD, et al. Evaluation of adipose-derived stromal vascular fraction or bone marrow-derived mesenchymal stem cells for treatment of osteoarthritis. J Orthop Res. 2009;27(12):1675–80. https://doi.org/10.1002/jor.20933.
Gao B, Wang Z, Borg T. 96-04: an MEA-based stem cell-Cardiomyocyte Coculture model for studying electrical signal propagation after stem cell transplantation. EP Europace. Narnia. 2016;18(suppl_1):i60–i60. https://doi.org/10.1093/europace/18.suppl_1.i60b.
Gao LR, et al. Intracoronary infusion of Wharton’s jelly-derived mesenchymal stem cells in acute myocardial infarction: double-blind, randomized controlled trial. BioMed Central (BMC). BioMed central ltd. 2015;13(1). https://doi.org/10.1186/s12916-015-0399-z.
De Girolamo L, et al. Treatment of chondral defects of the knee with one step matrix-assisted technique enhanced by autologous concentrated bone marrow: in vitro characterisation of mesenchymal stem cells from iliac crest and subchondral bone. Injury. 2010;41(11):1172–7. https://doi.org/10.1016/j.injury.2010.09.027.
Guijarro D, et al. Intramyocardial transplantation of mesenchymal stromal cells for chronic myocardial ischemia and impaired left ventricular function: results of the MESAMI 1 pilot trial. Int J Cardiol. Elsevier Ireland Ltd. 2016;209:258–65. https://doi.org/10.1016/j.ijcard.2016.02.016.
Guo X, et al. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: new regulators and its implications. Stem Cell Res Ther. BioMed Central. 2018;9(1):44. https://doi.org/10.1186/s13287-018-0773-9.
Gupta PK, et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. BioMed Central Ltd. 2016;18(1). https://doi.org/10.1186/s13075-016-1195-7.
Harding J, Roberts RM, Mirochnitchenko O. Large animal models for stem cell therapy. Stem Cell Res Ther. BioMed Central. 2013;4(2):23. https://doi.org/10.1186/SCRT171.
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (Prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–86. https://doi.org/10.1016/j.jacc.2009.06.055.
Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez D, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA - Journal of the American Medical Association. 2012;308(22):2369–79. https://doi.org/10.1001/jama.2012.25321.
Hare JM, DiFede D, Rieger AC, Florea V, Landin AM, el-Khorazaty J, et al. Randomized comparison of allogeneic versus autologous Mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J Am Coll Cardiol. Elsevier USA. 2017;69(5):526–37. https://doi.org/10.1016/j.jacc.2016.11.009.
Hatsushika D, Muneta T, Nakamura T, Horie M, Koga H, Nakagawa Y, et al. Repetitive allogeneic intraarticular injections of synovial mesenchymal stem cells promote meniscus regeneration in a porcine massive meniscus defect model. Osteoarthr Cartil. 2014;22(7):941–50. https://doi.org/10.1016/j.joca.2014.04.028.
Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–22. https://doi.org/10.1161/CIRCRESAHA.110.222703.
Henry TD, Pepine CJ, Lambert CR, Traverse JH, Schatz R, Costa M, et al. The Athena trials: autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter Cardiovasc Interv. 2017;89(2):169–77. https://doi.org/10.1002/ccd.26601.
Henze, D. A. and Urban, M. O. Large animal models for pain therapeutic development, Translational Pain Research: From Mouse to Man. CRC Press/Taylor & Francis. (2010) Available at: http://www.ncbi.nlm.nih.gov/pubmed/21882470 (Accessed: 22 September 2019).
Houtgraaf JH, et al. First experience in humans using adipose tissue-derived regenerative cells in the treatment of patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2012:539–40. https://doi.org/10.1016/j.jacc.2011.09.065.
Huang X, et al. Promoted chondrogenesis of cocultured chondrocytes and mesenchymal stem cells under hypoxia using in-situ forming degradable hydrogel scaffolds. Biomacromolecules. 2018;19(1):94–102. https://doi.org/10.1021/acs.biomac.7b01271.
Ismail S, O’Brien T, Barry F. The cardioprotective effect of MSC secreted protein in an in vitro model of myocardial injury: the mechanistic insight. Heart. BMJ publishing group ltd and British cardiovascular society. 2014;100. https://doi.org/10.1136/heartjnl-2014-306118.188.
Jimenez PA, Glasson SS, Trubetskoy OV, Haimes HB. Spontaneous osteoarthritis in Dunkin Hartley guinea pigs: histologic, radiologic, and biochemical changes. Lab Anim Sci. 1997;47(6):598–601.
Karantalis V, DiFede D, Gerstenblith G, Pham S, Symes J, Zambrano JP, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (PROMETHEUS) trial. Circ Res. Lippincott Williams and Wilkins. 2014;114(8):1302–10. https://doi.org/10.1161/CIRCRESAHA.114.303180.
Kastrup J, Haack-Sørensen M, Juhl M, Harary Søndergaard R, Follin B, Drozd Lund L, et al. Cryopreserved off-the-shelf allogeneic adipose-derived stromal cells for therapy in patients with ischemic heart disease and heart failure—a safety study. Stem Cells Transl Med. John Wiley and Sons Ltd. 2017;6(11):1963–71. https://doi.org/10.1002/sctm.17-0040.
Kehoe O, et al. Intra-articular injection of mesenchymal stem cells leads to reduced inflammation and cartilage damage in murine antigen-induced arthritis. J Transl Med. BioMed central. 2014;12(1):157. https://doi.org/10.1186/1479-5876-12-157.
Kim TW, et al. Direct coculture of human chondrocytes and synovium-derived stem cells enhances in vitro chondrogenesis. Cell J. Royan Institute. 2018;20(1):53–60. https://doi.org/10.22074/cellj.2018.5025.
Kong L, et al. Role of mesenchymal stem cells in osteoarthritis treatment. J OrthopTransl. Elsevier (Singapore) Pte ltd. 2017:89–103. https://doi.org/10.1016/j.jot.2017.03.006.
Kuyinu EL, et al. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res. BioMed Central. 2016;11:19. https://doi.org/10.1186/s13018-016-0346-5.
Lamo-Espinosa JM, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016, 246;14(1). https://doi.org/10.1186/s12967-016-0998-2.
Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Núñez-Córdoba JM, López-Elío S, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J Transl Med BioMed Central Ltd. 2018;16:1–5. https://doi.org/10.1186/s12967-018-1591-7.
Lee HW, et al. Effects of intracoronary administration of autologous adipose tissue-derived stem cells on acute myocardial infarction in a porcine model. Yonsei Med J. Yonsei University College of Medicine. 2015;56(6):1522–9. https://doi.org/10.3349/ymj.2015.56.6.1522.
Li, J. J. et al. (2019) ‘Stem cell-derived extracellular vesicles for treating joint injury and osteoarthritis’, Nanomaterials MDPI AG doi: https://doi.org/10.3390/nano9020261.
Lim M, et al. Intravenous injection of allogeneic umbilical cord-derived multipotent mesenchymal stromal cells reduces the infarct area and ameliorates cardiac function in a porcine model of acute myocardial infarction. Stem Cell Res Ther. BioMed Central Ltd. 2018;9(1):129. https://doi.org/10.1186/s13287-018-0888-z.
López Y, Lutjemeier B, Seshareddy K, Trevino EM, Hageman KS, Musch TI, et al. Wharton’s jelly or bone marrow mesenchymal stromal cells improve cardiac function following myocardial infarction for more than 32 weeks in a rat model: a preliminary report. Current stem cell research & therapy. 2013;8(1):46–59.
Lu L, Liu M, Sun R, Zheng Y, Zhang P. Myocardial infarction: symptoms and treatments. Cell Biochem Biophys. 2015;72(3):865–7. https://doi.org/10.1007/s12013-015-0553-4.
Macrì S, et al. The directive 2010/63/EU on animal experimentation may skew the conclusions of pharmacological and behavioural studies. Sci Rep. 2013;3(1):2380. https://doi.org/10.1038/srep02380.
Mak J, et al. Intra-articular injection of synovial mesenchymal stem cells improves cartilage repair in a mouse injury model. Sci Rep. Nature Publishing Group. 2016;6(1):23076. https://doi.org/10.1038/srep23076.
Mandal BB, et al. Stem cell-based meniscus tissue engineering. Tissue Eng Part A. Mary Ann Liebert, Inc. 2011;17(21-22):2749–61. https://doi.org/10.1089/ten.TEA.2011.0031.
Markmee R, Aungsuchawan S, Narakornsak S, Tancharoen W, Bumrungkit K, Pangchaidee N, et al. Differentiation of mesenchymal stem cells from human amniotic fluid to cardiomyocyte-like cells. Mol Med Rep. Spandidos Publications. 2017;16(5):6068–76. https://doi.org/10.3892/mmr.2017.7333.
Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, et al. Umbilical cord-derived Mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8(3):215–24. https://doi.org/10.1002/sctm.18-0053.
Mathiasen AB, et al. Bone marrow-derived mesenchymal stromal cell treatment in patients with severe ischaemic heart failure: a randomized placebo-controlled trial (MSC-HF trial). Eur Heart J. Oxford University Press. 2015;36(27):1744–53. https://doi.org/10.1093/eurheartj/ehv136.
Mazo M, et al. Treatment of reperfused ischemia with adipose-derived stem cells in a preclinical swine model of myocardial infarction. Cell Transplant. 2012;21(12):2723–33. https://doi.org/10.3727/096368912X638847.
McCall FC, et al. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc. 2012;7(8):1479–96. https://doi.org/10.1038/nprot.2012.075.
McGovern JA, Griffin M, Hutmacher DW. Animal models for bone tissue engineering and modelling disease. Dis Model Mech. Company of Biologists. 2018;11(4). https://doi.org/10.1242/dmm.033084.
Miki S, Takao M, Miyamoto Wataru. Intra-articular injection of synovium-derived mesenchymal stem cells with hyaluronic acid can repair articular cartilage defects in a canine model. Stem Cell Res Ther. 2015;5(11).
Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, et al. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10(4):459–66. https://doi.org/10.1016/j.bbmt.2007.12.115.
Mohseni, J., Kazemi T., Maleki M.H., Beydokhti H. A systematic review on the prevalence of acute myocardial infarction in Iran. Heart views : the official journal of the Gulf Heart Association. Wolters Kluwer -- Medknow Publications, (2017) 18(4), pp. 125–132. doi: https://doi.org/10.4103/HEARTVIEWS.HEARTVIEWS_71_17.
Mokbel AN, et al. Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord. 2011;12. https://doi.org/10.1186/1471-2474-12-259.
Moran CJ, et al. The benefits and limitations of animal models for translational research in cartilage repair. J Exp Orthop. Springer. 2016;3(1):1. https://doi.org/10.1186/s40634-015-0037-x.
Murphy JM, et al. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. John Wiley & Sons, Ltd. 2003;48(12):3464–74. https://doi.org/10.1002/art.11365.
Musialek P, Mazurek A, Jarocha D, Tekieli L, Szot W, Kostkiewicz M, et al. Myocardial regeneration strategy using Wharton’s jelly mesenchymal stem cells as an off-the-shelf “unlimited” therapeutic agent: results from the acute myocardial infarction first-in-man study. Postepy w Kardiologii Interwencyjnej Termedia Publishing House Ltd. 2015;11(2):100–7. https://doi.org/10.5114/pwki.2015.52282.
Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112(8):1128–35. https://doi.org/10.1161/CIRCULATIONAHA.104.500447.
Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr Cartil. NIH Public Access. 2013;21(9):1145–53. https://doi.org/10.1016/j.joca.2013.03.018.
Nerurkar NL, et al. Homologous structure-function relationships between native fibrocartilage and tissue engineered from MSC-seeded nanofibrous scaffolds. Biomaterials. NIH Public Access. 2011;32(2):461–8. https://doi.org/10.1016/j.biomaterials.2010.09.015.
Nuki G. Osteoarthritis: a problem of joint failure. Z Rheumatol. 1999;58(3):142–7.
Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95(12):1535–41. https://doi.org/10.1097/TP.0b013e318291a2da.
Pabbruwe MB, Kafienah W, Tarlton JF, Mistry S, Fox DJ, Hollander AP. Repair of meniscal cartilage white zone tears using a stem cell/collagen-scaffold implant. Biomaterials. 2010;31(9):2583–91. https://doi.org/10.1016/j.biomaterials.2009.12.023.
Park SA, Reilly CM, Wood JA, Chung DJ, Carrade DD, Deremer SL, et al. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy. 2013;15(12):1498–1510.
Pers Y-M, Rackwitz L, Ferreira R, Pullig O, Delfour C, Barry F, et al. Adipose mesenchymal stromal cell-based therapy for severe osteoarthritis of the knee: a phase I dose-escalation trial. Stem Cells Transl Med. 2016;5(7):847–56. https://doi.org/10.5966/sctm.2015-0245.
Poole R, Blake S, Buschmann M, Goldring S, Laverty S, Lockwood S, et al. Recommendations for the use of preclinical models in the study and treatment of osteoarthritisOsteoarthr Cartil. 2010;18:S10–6. https://doi.org/10.1016/j.joca.2010.05.027.
Qayyum AA, et al. Adipose-derived stromal cells for treatment of patients with chronic ischemic heart disease (MyStromalCell trial): a randomized placebo-controlled study. Stem Cells Int. Hindawi Limited. 2017;2017. https://doi.org/10.1155/2017/5237063.
Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106(33):14022–7. https://doi.org/10.1073/pnas.0903201106.
Reddy K. Recent advances in the diagnosis and treatment of acute myocardial infarction. World J Cardiol. 2015;7(5):243. https://doi.org/10.4330/wjc.v7.i5.243.
Rodrigo SF, van Ramshorst J, Hoogslag GE, Boden H, Velders MA, Cannegieter SC, et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res. 2013;6(5):816–25. https://doi.org/10.1007/s12265-013-9507-7.
Ruane, J. (2019) Investigation of mesenchymal stem cell therapy for the treatment of osteoarthritis of the knee—full text view— ClinicalTrials.gov . Available at: https://clinicaltrials.gov/ct2/show/study/NCT02958267 (Accessed: 14 January 2020).
Rumana N, Kita Y, Turin TC, Murakami Y, Sugihara H, Morita Y, et al. Trend of increase in the incidence of acute myocardial infarction in a Japanese population: Takashima AMI registry, 1990-2001. Am J Epidemiol. 2008;167(11):1358–64. https://doi.org/10.1093/aje/kwn064.
Saleh M, Ambrose JA. Understanding myocardial infarction. F1000Res. Faculty of 1000 Ltd. 2018;7. https://doi.org/10.12688/f1000research.15096.1.
Salvatore CA, Hershey JC, Corcoran HA, Fay JF, Johnston VK, Moore EL, et al. Pharmacological Characterization of MK-0974 [ N -[(3 R ,6 S )-6-(2,3-difluorophenyl)-2-oxo-1-(2,2,2-trifluoroethyl)azepan-3-yl]-4-(2-oxo-2,3-dihydro-1 H -imidazo[4,5- b ]pyridin-1-yl)piperidine-1-carboxamide], a potent and orally active calcitonin gene-related peptide receptor antagonist for the treatment of migraine. J Pharmacol Exp Ther. 2008;324(2):416–21. https://doi.org/10.1124/jpet.107.130344.
Samson DJ, et al. Treatment of primary and secondary osteoarthritis of the knee. Evid Rep Technol Assess. 2007;157:1–157.
Schuleri KH, et al. Autologous mesenchymal stem cells produce reverse remodelling in chronic ischaemic cardiomyopathy. Eur Heart J. 2009;30(22):2722–32. https://doi.org/10.1093/eurheartj/ehp265.
Shadmanfar S, et al. Intra-articular knee implantation of autologous bone marrow–derived mesenchymal stromal cells in rheumatoid arthritis patients with knee involvement: results of a randomized, triple-blind, placebo-controlled phase 1/2 clinical trial. Cytotherapy. Elsevier B.V. 2018;20(4):499–506. https://doi.org/10.1016/j.jcyt.2017.12.009.
Shapiro SA, et al. A prospective, single-blind, placebo-controlled trial of bone marrow aspirate concentrate for knee osteoarthritis. Am J Sports Med, SAGE Publications Inc. 2017;45(1):82–90. https://doi.org/10.1177/0363546516662455.
Sharma AR, Jagga S, Lee SS, Nam JS. Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci. Multidisciplinary Digital Publishing Institute (MDPI). 2013;14(10):19805–30. https://doi.org/10.3390/ijms141019805.
Soler R, et al. Final results of a phase I–II trial using ex vivo expanded autologous mesenchymal stromal cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. Elsevier B.V. 2016;23(4):647–54. https://doi.org/10.1016/j.knee.2015.08.013.
Solinas P, Isola M, Lilliu MA, Conti G, Civolani A, Demelia L, et al. Animal models are reliably mimicking human diseases? A morphological study that compares animal with human NAFLD. Microsc Res Tech. 2014;77(10):790–6. https://doi.org/10.1002/jemt.22401.
Song F, et al. Comparison of the efficacy of bone marrow mononuclear cells and bone mesenchymal stem cells in the treatment of osteoarthritis in a sheep model. Int J Clin Exp Pathol. 2014;7(4):1415–26.
Song Y, et al. ‘Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. Future Medicine Ltd. 2018;13(3):295–307. https://doi.org/10.2217/rme-2017-0152.
Succar P, et al. ‘Priming adipose-derived mesenchymal stem cells with hyaluronan alters growth kinetics and increases attachment to articular cartilage. Stem Cells Int Hindawi. 2016;2016:1–13. https://doi.org/10.1155/2016/9364213.
Swindle MM, et al. Swine as models in biomedical research and toxicology testing. Vet Pathol. SAGE PublicationsSage CA: Los Angeles, CA. 2012;49(2):344–56. https://doi.org/10.1177/0300985811402846.
Szaraz P, et al. <em>In Vitro</em> Differentiation of human mesenchymal stem cells into functional cardiomyocyte-like cells. J Vis Exp. 2017;(126). https://doi.org/10.3791/55757.
Taghiyar, L., Gourabi, H. and Eslaminejad, M. B. (2010) Autologous transplantation of mesenchymal Stem cells (MSCs) and scaffold in full-thickness articular cartilage—full text view - ClinicalTrials.gov.
Tang Y, Pan ZY, Zou Y, He Y, Yang PY, Tang QQ, et al. A comparative assessment of adipose-derived stem cells from subcutaneous and visceral fat as a potential cell source for knee osteoarthritis treatment. J Cell Mol Med. 2017;21(9):2153–62. https://doi.org/10.1111/jcmm.13138.
Thomas B, Bhat K, Mapara M. Rabbit as an animal model for experimental research. Dent Res J. Medknow. 2012;9(1):111. https://doi.org/10.4103/1735-3327.92960.
Tseng WJ, et al. Treatment of osteoarthritis with collagen-based scaffold: a porcine animal model with xenograft mesenchymal stem cells. Histol Histopathol. 2018;33(12):1271–86. https://doi.org/10.14670/HH-18-013.
Ude CC, Sulaiman SB, Min-Hwei N, Hui-Cheng C, Ahmad J, Yahaya NM, et al. Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PLoS One. Public Libr Sci. 2014;9(6). https://doi.org/10.1371/journal.pone.0098770.
Wang L, et al. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. Mary Ann Liebert Inc. 2009;15(8):2259–66. https://doi.org/10.1089/ten.tea.2008.0393.
Wang S, et al. Human neonatal thymus mesenchymal stem cells promote neovascularization and cardiac regeneration. Stem Cells Int. Hindawi. 2018;2018:1–7. https://doi.org/10.1155/2018/8503468.
White IA, Sanina C, Balkan W, Hare JM. Mesenchymal stem cells in cardiology. Journal of Molecular Biology and Methods (MBM). NIH Public Access. 2016;1416:55–87. https://doi.org/10.1007/978-1-4939-3584-0_4.
Williams AR, et al. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole-chamber remodeling. J Am Heart Assoc. 2013;2(3):e000140. https://doi.org/10.1161/JAHA.113.000140.
Xia T, Yu F, Zhang K, Wu Z, Shi D, Teng H, et al. The effectiveness of allogeneic mesenchymal stem cells therapy for knee osteoarthritis in pigs. Ann Transl Med. 2018;6(20):404–404.
Xiang M, He AN, Wang JA, Gui C. Protective paracrine effect of mesenchymal stem cells on cardiomyocytes. J Zhejiang Univ Sci B. Zhejiang University Press. 2009;10(8):619–24. https://doi.org/10.1631/jzus.B0920153.
Zellner J, et al. Role of mesenchymal stem cells in tissue engineering of meniscus. J Biomed Mater Res A. 2010;9999A(4):NA-NA. https://doi.org/10.1002/jbm.a.32796.
Ziegler A, Gonzalez L, Blikslager A. Large animal models: the key to translational discovery in digestive disease research. Cell Mol Gastroenterol Hepatol. Elsevier. 2016;2(6):716–24. https://doi.org/10.1016/j.jcmgh.2016.09.003.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 761214. The material presented and views expressed here are the responsibility of the author(s) only. The EU Commission takes no responsibility for any use made of the information set out.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hotham, W.E., Henson, F.M.D. The use of large animals to facilitate the process of MSC going from laboratory to patient—‘bench to bedside’. Cell Biol Toxicol 36, 103–114 (2020). https://doi.org/10.1007/s10565-020-09521-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-020-09521-9