Abstract
Insects live in a dangerous world and may fall prey to a wide variety of predators, encompassing multiple taxa. As a result, selection may favour defences that are effective against multiple predator types, or target-specific defences that can reduce predation risk from particular groups of predators. Given the variation in sensory systems and hunting tactics, in particular between vertebrate and invertebrate predators, it is not always clear whether defences, such as chemical defences, that are effective against one group will be so against another. Despite this, the majority of research to date has focused on the role of a single predator species when considering the evolution of defended prey. Here we test the effectiveness of the chemical defences of the wood tiger moth, a species previously shown to have defensive chemicals targeted towards ants, against a common invertebrate predator: spiders. We presented both live moths and artificial prey containing their defensive fluids to female Trichonephila senegalensis and recorded their reactions. We found that neither of the moth’s two defensive fluids were able to repel the spiders, and confirmed that methoxypyrazines, a major component of the defences of both the wood tiger moth and many insect species, are ineffective against web-building spiders. Our results highlight the variability between predator taxa in their susceptibility to chemical defences, which can in part explain the vast variation in these chemicals seen in insects, and the existence of multiple defences in a single species.
Similar content being viewed by others
Introduction
Almost all organisms are at risk of predation during some stage of their life. This strong selective pressure to avoid injury or death at the hands of a hungry predator has resulted in a diverse array of anti-predator strategies. Of these, chemical defence is one of the most taxonomically widespread (Speed et al. 2012). While there has been a trend in the past decades to use a greater variety of predators to test the effectiveness of chemical defences, ants and birds still predominate in such studies. Although both groups are likely to be important predators in many environments, the huge variability in both sensory systems, and thus susceptibility to chemical defences, shown by predatory species means that over-reliance on a few taxa may produce misleading results (Zvereva and Kozlov 2016). In addition, individual prey species are likely to fall prey to multiple predator species, thus it is important to test a variety of ecologically relevant predators to fully understand the strength of a species’ chemical defences (Endler and Mappes 2004).
The wood tiger moth (Arctia plantaginis, Lepidoptera, Arctiidae) is a capital breeding species with a Holarctic distribution (Hegna et al. 2015). It is aposematic, displaying colourful hindwings paired with two types of defensive fluids which it secretes when attacked. These fluids, which are costly both to produce and deploy (Burdfield-Steel et al. 2018b, Lindstedt et al. in review), have been found to be targeted towards particular types of predators. The neck fluids, which are released from specialised glands during attacks to the head of the moth, deter bird predators, such as blue tits, but are ineffective against ants, while the abdominal fluids, produced from the anus when the moth is startled or disturbed, are aversive to wood ants but not birds (Rojas et al. 2017). The main chemical compounds used by the moths appear to be methoxypyrazines. In particular, 2-sec-butyl-3-methoxypyrazine (SBMP) and 2-isobutyl-3-methoxypyrazine (IBMP) have been found in the neck fluids of the moths (Burdfield-Steel et al. 2018a) and elicit similar levels of aversion in wild birds (Rojas et al. 2017 and Burdfield-Steel et al. unpublished data). In addition, 2-isopropyl-3-methoxypyrazine (IPMP) has been detected in the abdominal fluids of some moths, and may therefore play a role in defence against invertebrates (A. Winters, pers. com.). Pyrazines, and methoxypyrazines in particular, are used as part of chemical defences and displays by many insects and other invertebrates (Guilford et al. 1987; Moore et al. 1990; Kögel et al. 2012) including tiger moths (Rothschild et al. 1984). Their widespread use has led to the suggestion that they may act as alerting or warning signals, much like bright colouration, indicating the presence of stronger chemical defences to more chemical or odour-oriented predators (Moore et al. 1990). This is supported by the finding that pyrazines can facilitate predator learning (Rowe and Guilford 1996) and induce aversions to visually conspicuous prey (Lindström et al. 2001) in birds. While the strong methoxypyrazine-produced odour of the neck fluids does appear to interact with the visual warning signal in the wood tiger moth (Rojas et al. 2019), these pyrazines also appear to function as the defence itself, as they are distasteful to wild-caught birds (Rojas et al. 2017) even in the absence of any other cues or chemicals. However the role of IPMP, if any, in the abdominal fluids remains unknown.
The presence of multiple forms of chemical defence highlights the dual predation pressure, from both vertebrate and invertebrate predators, shaping the antipredator adaptations in this species. However, their effectiveness against one of the most numerous types of invertebrate predators, spiders, remains untested. Web-building spiders are an important class of predators in terrestrial environments (Nyffeler and Birkhofer 2017). They have previously been shown to reject insect prey defended by different chemicals including the moths Empyreuma pugione (Petschenka et al. 2011) and Utetheisa ornatrix (Eisner 1982) (both Arctiidae). Observations of moths caught in spider webs during enclosure experiments (Gordon et al. 2015) suggest that spiders pose a real threat to this species (S. Gordon, pers. coms). Orb-weaving spiders of the genus Nephila (recently renamed to Trichonephila, Kuntner et al. 2018), are commonly used as predators in experiments testing Lepidoptera defences (Vasconcellos-Neto and Lewinsohn 1984), in particular the ithomiine butterflies (Brown 1984; Trigo et al. 1996; Orr et al. 1996). Trichonephila senegalensis is a particularly useful species given its willingness to attack and feed on “artificial prey” in the form of gelatin capsules. This allows for precise manipulation of the chemical contents of the prey, without any changes to external cues.
Here we test if the wood tiger moth is palatable to a model spider predator, Trichonephila senegalensis (Araneae, Araneidae). We predict that, as a result of their chemical defences, wood tiger moths will show higher survival than undefended flies when presented to spiders. Furthermore, we use an artificial prey assay to determine if the moths’ two defensive fluids, and the methoxypyrazines they contain, are effective in deterring spiders. In keeping with previous findings using ants, we predict that the neck fluids will not deter spiders, but that the abdominal defensive fluids will.
Methods
We used adult female Trichonephila senegalensis bred and raised at Universität Hamburg from a source population stemming from spiders collected in Ndumo South Africa in 2015 for Exp. 1 and 2, and in 2017 for Exp. 3 (permit 7,902,215,171,083 from Ezemvelo KZN Wildlife). Spiders were raised from eggs under standardised conditions with temperatures between 24 and 26 °C (although higher temperatures can occur if the cooling system fails) and a humidity between 50 and 60%. After hatching from the egg-sacs, juveniles were transferred individually to translucent cups of sizes between 250 ml and 1 l depending on spider size. Cups were scratched inside to facilitate web attachment and fitted with cotton wool stuffed in a hole of the cup bottom. Spiders were fed with Drosophila and flies (Calliphora vicina) twice a week and their webs were misted with water 5 days per week. Each spider was naïve to prey that contain defensive chemicals. Adult female spiders were placed in acrylic frames (60 × 60 cm) and allowed to build a web. Each spider was food deprived for a minimum of 2 days prior to the trials.
Wood tiger moths were obtained from a laboratory stock established in 2011, from wild moths collected from central and southern Finland, and reared at the University of Jyväskylä (Finland) under natural light conditions and temperature ca. 23 °C. After eclosion adult moths were kept at 7 °C and provided with water. As wood tiger moths are capital breeders, adults do not eat. Their chemical defences take the form of “neck fluids”, secreted from the prothoracic (cervical) glands, and “abdominal fluids”, released from the abdominal tract. Fluids for experiment 2 were collected in 2016 from approximately 36 individuals between 0 and 3 days post-eclosion. Prior to sampling all moths were removed from the climate chamber in which they were stored and sprayed with water. They were then given one hour at room temperature to drink and become active. Neck fluids were sampled by pinching the moth just below the prothoracic glands with a pair of tweezers. This stimulated the release of the fluid, which was then collected with 10 µl glass capillaries. The abdominal fluid was sampled by gently squeezing the moth’s abdomen with tweezers and collected in the same manner. The fluids were then pooled into groups of three moths and kept at − 20 °C prior to injection into the artificial prey.
Experiment 1
Each spider was offered a moth and a fly (Calliphora vicina) in a randomised order and their behaviour recorded. Prey were chilled to temporarily reduce activity and placed in the web of the spider. All prey were weighed prior to the start of the trial and, where possible, the weights were matched such that spiders that received larger moths also received larger flies. Prey were placed in the web approximately 5 cm from the hub. We then recorded the time taken for the spider to attack the prey, the amount of time spent on activities such as wrapping and feeding, and the final weight of the prey once dropped from the web. Spiders were given 2 h to attack prey in the web. Prey not attacked after this time were considered to have “survived”. If they did attack the prey, spiders were observed for 2 h, or until the prey had been dropped from the web.
Experiment 2
Artificial prey were made using gelatine capsules (1.43 cm × 0.52 cm) filled with meat broth (1% meat extract, 5% starch) (see Fig. 1). Capsules were first coated with lard (heated to 50 °C then cooled to 23 °C) using a fine paint brush. They were then assembled and allowed to cool for 2 h before being injected and filled with meat broth. Control capsules were injected with 190 µl of broth, treatment capsules were injected with a mixture of the broth and defensive fluids from three adult moths. This ratio of broth to defensive fluid was chosen given that the capsules were, on average, slightly less than three times the weight of the moths. Three capsules (control, neck and abdominal) were offered to the spiders sequentially in a randomised order. As the capsules were larger than the insect prey used in experiment one, spiders were given 24 h between capsules to ensure they were motivated to attack. Capsules were introduced to the web using tweezers and then vibrated using a modified electric toothbrush until the spider attacked. For this reason time to attack was not recorded in these trials. Spiders were given 2 h to feed after which the capsule was removed from the web and re-weighed. Previous studies suggest that weight lost through evaporation during these trials is negligible (Petschenka et al. 2011), however the ambient temperature of the laboratory was particularly high during this experiment (25–31 °C) therefore the temperature of the room during each trial was recorded.
Experiment 3
Following a similar protocol as experiment 2, spiders were presented with four capsules containing either control (as before), 2-sec-butyl-3-methoxypyrazine (SBMP), 2-isobutyl-3-methoxypyrazine (IBMP) or 2-isopropyl-3-methoxypyrazine (IPMP). All pyrazines were bought from Sigma-Aldrich. Presentation was done over a period of 4 days, with the spiders receiving one capsule each day in a randomised order. Spiders initially received concentrations of 1 µl of pyrazine in 1 ml meat broth, as this is the upper range found naturally in the neck fluids of the moths. The experiment was then repeated, using different spiders, one and 2 weeks later with concentrations of 5 µl/ml (week 2) and 10 µl/ml (week 3). The weight of the capsule before and after the trials was recorded. In this experiment spiders were allowed to feed on the artificial prey until they chose to stop, therefore feeding duration was also recorded.
Statistical analysis
As all three experiments involved repeated measures of individual spiders presented with different prey types, all data, except the likelihood to feed in experiment 3, were analysed using generalised estimating equations (GEEs) using the package ‘geepack’ (Højsgaard et al. 2005) with prey type and sequence order as explanatory variables. GEEs are useful for analysing non-normally distributed data such as ours and make fewer assumptions than mixed-effect models (Pekar and Brabec 2018). In experiment 1, the proportion of weight lost from each of the prey, along with behavioural measures such as time taken to attack, time spent wrapping the prey and time spent feeding, were analysed. In experiment two the proportion of weight lost from the capsules was analysed. In experiment 3 the percentage of weight lost from each of the artificial prey, and the feeding duration were analysed. The likelihood that a spider would feed on a capsule was analysed using a generalized linear mixed-effects model with a binomial distribution with feeding (yes or no) as the dependent variable and treatment type as the explanatory variable. Each concentration of each pyrazine was considered as a separate treatment. All analyses were done in R 3.4.4. (R Development Core Team 2008).
Results
Experiment 1
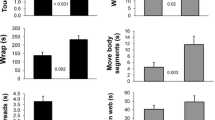
17 T. senegalensis were tested with live insect prey. Of the 17 moths presented to the spiders only two survived. One female moth was attacked then cut from the web and one male moth was never attacked. All the flies presented were eaten. The spiders took longer to attack, wrap, and eat moth prey (GEE: Attack: W = 8.08, p = 0.005, Wrap: W = 12.84, p = 0.0003 and Feed: W = 4.48, p = 0.034) and they ate a smaller proportion of moths compared to flies (W = 13.00, p = 0.0003, Fig. 2).
Comparisons of spider behaviour with fly and moth prey. Top left—the proportion of prey weight lost during spider feeding. Top right—the time, in seconds, spiders spent feeding on the two prey types. Bottom left—the time, in seconds, taken by spiders to attack the different prey types. Bottom right—the time, in seconds, spiders spent wrapping different prey types. Thick lines indicate medians and boxes show upper and lower quartiles. Whiskers indicate 1.5 times the interquartile range
Experiment 2
12 T. senegalensis were tested with the three artificial prey. Only two capsules were cut from the web during the course of the experiment, one control capsule and one capsule containing neck fluid. There was no significant effect of capsule type (GEE: W = 3.54, p = 0.06 and W = 0.11, p = 0.75 for neck and abdominal fluid treatments respectively, Fig. 3) or presentation order (W = 0.74, p = 0.39) on the weight lost from the capsules during spider feeding.
Experiment 3
A total of 44 T. senegalensis were tested with the pure pyrazines across 45 trials, 15 with concentrations of 1 µl/ml, 15 with 5 µl/ml and 15 with 10 µl/ml. One spider was included in two concentration treatments: 1 µl/ml and 10 µl/ml. The spiders fed on the capsules in the majority (85%) of the trials. The likelihood that a spider would feed was not affected by any of the treatments (p > 0.05 for all treatments, Table 1) although it did reduce with subsequent presentations (GLMM: z = − 2.37, p = 0.0176). Of the 180 capsules presented to spiders only eight were cut from the web prior to feeding, four from the 1 µl/ml and four from the 5 µl/ml concentrations. The order in which the capsules were presented to the spiders did significantly affect the amount of weight lost, with those presented later losing less weight (W = 7.96, p = 0.0048) but not the amount of time spent feeding (W = 3.45, p = 0.0633). When compared to the controls, none of the treatments affected the amount of weight lost from the capsules (W = 1.18, p = 0.278 for SBMP, W = 0.03, p = 0.8616 for IBMP and W = 1.08, p = 0.299 for IPMP, Fig. 4) or the time spent feeding (W = 2.18, p = 0.1400, W = 1.16, p = 0.2808 and W = 0.54, p = 0.4627 for SBMP, IBMP and IPMP respectively, Fig. 5). The concentration of pyrazine in the capsules did not affect the amount of weight lost (W = 0.47, p = 0.4911), but it did significantly affect time spent feeding (W = 8.74, p = 0.0031) as higher concentrations were fed on by the spiders for longer (Fig. 5).
The proportion of weight lost by artificial prey capsules during spider feeding in experiment 3. IBMP = 2-isobutyl-3-methoxypyrazine. SBMP = 2-sec-butyl-3-methoxypyrazine. IPMP = 2-isopropyl-3-methoxypyrazine. Thick lines indicate medians and boxes show upper and lower quartiles. Whiskers indicate 1.5 times the interquartile range
The time (in seconds) spiders spent feeding on artificial prey capsules during experiment 3. IBMP = 2-isobutyl-3-methoxypyrazine. SBMP = 2-sec-butyl-3-methoxypyrazine. IPMP = 2-isopropyl-3-methoxypyrazine. Thick lines indicate medians and boxes show upper and lower quartiles. Whiskers indicate 1.5 times the interquartile range
Discussion
While the defensive fluids of the wood tiger moth have been shown to deter both ant and bird predators (Rojas et al. 2017), they were ineffective against model spider predators. Almost all live moths offered to the spiders were consumed, and artificial prey treated with both neck and abdominal fluids were eaten at the same rate as control prey. Furthermore, methoxypyrazines, a key component of the moth’s chemical defences (Burdfield-Steel et al. 2018b), failed to deter spiders even at very high concentrations.
T. senegalensis have previously been shown to reject other moth species, such as Empyreuma pugione, and even flies, when those species contain chemicals the spiders find unpalatable, such as cardenolides (Petschenka et al. 2011). However, we found no evidence that wood tiger moths were unpalatable to T. senegalensis. While the spiders did take longer to attack wood tiger moths in comparison to their usual fly prey, this may have been because the moths moved less in the web than the flies. Alternatively, it may be that the spiders were more cautious when attacking unfamiliar prey (but see Bramer et al. 2018). In addition, the spiders spent more time wrapping the moths, and ate a smaller proportion of their body weight compared to the flies, although this was probably a result of the larger wings of the moths. When the defensive fluids of the moths were presented to the spiders in artificial prey they had no impact on spider feeding behaviour.
Capsule assays with three pure methoxypyrazines confirmed their ineffectiveness as defences against spiders, even at very high doses. For comparison, the two methoxypyrazines found in the neck fluids of the wood tiger moth, IBMP and SBMP, show clear deterrent effects on birds at concentrations of less than 1 µl/ml (Rojas et al. 2017, Burdfield-Steel et al. unpublished data), which is also towards the high end of the range found in the defensive fluids of the moths (Burdfield-Steel et al. ; 2018b). In fact, far from acting as deterrents, 1 µl/ml concentrations of SBMP and IPBP actually appeared to increase spider feeding time (Fig. 5). This is in keeping with previous work, which found methoxypyrazines, in particular IPMP, to be ineffective at repelling the smaller European bridge spider Larinioides sclopetarius (Priebusch 2012) and that Harmonia axyridis ladybirds, which have been shown to contain both IPMP and SBMP (Kögel et al. 2012), are palatable to European orb-web spiders (Sloggett 2010). The finding that methoxypyrazines do not confer protection from spiders supports the conclusions of previous work using ants, which suggested that these pyrazines do not provide protection against invertebrate predators (Rojas et al. 2017, but see Brown and Moore, 1979 for more on the role of pyrazines in intra-specific communication in ants). The biologically active components of the abdominal fluids are still unknown, although preliminary analysis suggests they are not toxic (Rojas et al. 2017).
This lack of protection from web-building spiders is in keeping with observations from the field and outdoor enclosure experiments where moths were observed caught in spider webs (Gordon, pers. com.). This could be due to the constraints of de novo production (Burdfield-Steel et al. 2018b), making it costly to produce compounds aversive to spiders, or may be because there is limited survival benefit gained from protection from this type of predator. While jumping spiders do occur in the range of the wood tiger moth, in Finland the species present are too small to be considered a risk to the wood tiger moth. Thus, potential arachnid predators are web-building spiders. While some web-building spiders will cut defended prey from their webs alive, it may be that the energy expended in escaping the spiders’ silk may already significantly decrease the future fitness of these short-lived, capital breeding moths to effectively zero. The results of a limited number of trials with the smaller European species L. sclopetarius suggest the moths are palatable even to smaller spiders, but that the size of the moths, and difficulties handling them, may be enough to provide some protection, as two individuals were released by bridge spiders (see Supplementary materials). In addition, while actively hunting spiders have been shown to learn to avoid aposematic prey (McIver and Lattin 1990) it is far less clear if web-building spiders can do the same (Bramer et al. 2018), particularly as they are unlikely to use visual cues, such as colour. Without avoidance learning these spiders can only taste-reject prey, which may also reduce the benefit of chemical defences against them. However, this explanation seems less likely given that some of the best evidence for the anti-predatory effect of compounds such as pyrrolizidine alkaloids and cardiac glycosides have come from web-building spiders (e.g. Brown 1984; Petschenka et al 2011).
An alternative explanation for this lack of protection is the source of these chemicals, which in the wood tiger moth are produced de novo (also known as autogenously) (Burdfield-Steel et al. 2018b). A recent meta-analysis found that chemical compounds synthesised de novo did not differ in the magnitude of their effects compared to those derived from the herbivore's food plants (Zvereva and Kozlov 2016). However, it could be that de novo defences are more targeted. Many of the best-known plant-derived defensive chemicals confer broad protection (for example cardiac glycosides, which have been shown to confer defence against vertebrates (Brower and Glazier 1975), insects (Berenbaum and Miliczky 1984), spiders (Petschenka et al. 2011) and even parasites (Tan et al. 2018)). It is also notable that the related species U. ornatrix, whose chemical defences (pyrrolizidine alkaloids) are effective at deterring spiders, does sequester these from its diet (Eisner 1982). Given the tendency to focus testing of putative chemical defences on a small group of predators it is hard to say if the level of targeting differs between sequestered versus de novo defences, although this may be an interesting topic for further investigation as more data on the effects of different chemical defences on a variety of predators becomes available.
In conclusion, the chemical defences of the wood tiger moth did not confer any protection from web-building spiders. This is in contrast to the hypothesis put forward by Moore et al (1990) that the odour of methoxypyrazines is a universal warning signal. Instead it seems that the effect of these chemicals is highly taxa-specific, even among chemically-oriented predators and that, for many chemically defended species, there is still someone who can eat you.
References
Berenbaum M, Miliczky E (1984) Mantids and milkweed bugs: efficacy of aposematic coloration against invertebrate predators. Am Midl Nat 111(1):64–68. https://doi.org/10.2307/2425543
Bramer C, Schweitzer C, Dobler S (2018) Cardenolide-defended milkweed bugs do not evoke learning in Nephila senegalensis spiders. Ethology 124:504–513
Brower L, Glazier S (1975) Localization of heart poisons in the monarch butterfly. Science 188(4183):19–25
Brown KS (1984) Adult-obtained pyrrolizidine alkaloids defend ithomiine butterflies against a spider predator. Nature 309:707–709
Burdfield-Steel E, Brain M, Rojas B, Mappes J (2019a) The price of safety: food deprivation in early life influences the efficacy of chemical defence in an aposematic moth. Oikos. https://doi.org/10.1111/oik.05420
Burdfield-Steel E, Pakkanen H, Rojas B, Galarza JA, Mappes J (2018b) De novo synthesis of chemical defences in an aposematic moth. J Insect Sci. https://doi.org/10.1093/jisesa/iey020
Eisner T (1982) For love of nature: exploration and discovery at biological field stations. Bioscience 32(5):321–326
Endler JA, Mappes J (2004) Predator mixes and the conspicuousness of aposematic signals. Am Nat 163(4):532–547
Gordon SP, Kokko H, Rojas B, Nokelainen O, Mappes J (2015) Colour polymorphism torn apart by opposing positive frequency-dependent selection, yet maintained in space. J Anim Ecol. https://doi.org/10.1111/1365-2656.12416
Guilford T, Nicol C, Rothschild M, Moore BP (1987) The biological roles of pyrazines: evidence for a warning odour function. Biol J Linn Soc 31:113–128
Hegna RH, Galarza JA, Mappes J (2015) Global phylogeography and geographical variation in warning coloration of the wood tiger moth (Parasemia plantaginis). J Biogeogr 42(8):1469–1481
Højsgaard S, Halekoh U, Yan J (2005) The R Package geepack for generalized estimating equations. J Stat Soft 15(2):1–11. https://doi.org/10.18637/jss.v015.i02
Kögel S, Eben A, Hoffmann C, Gross J (2012) Influence of diet on fecundity, immune defense and content of 2-isopropyl-3-methoxypyrazine in Harmonia axyridis pallas. J Chem Ecol 38:854. https://doi.org/10.1007/s10886-012-0139-1
Kuntner M, Hamilton CA, Cheng R-C, Gregorič M, Lupše N, Lokovšek T, Lemmon EM, Lemmon AR, Agnarsson I, Coddington JA, Bond JE (2018) Golden orb weavers ignore biological rules: phylogenomic and comparative analyses unravel a complex evolution of sexual size dimorphism. Syst Biol 68:555–572
Lindström L, Rowe C, Guilford T (2001) Pyrazine odour makes visually conspicuous prey aversive. Proc Biol Sci 268(1463):159–162
McIver JD, Lattin JD (1990) Evidence for aposematism in the plant bug Lopidea nigridea Uhler (Hemiptera: Miridae: Orthotylinae). Biol J Lin Soc 40:99–112
Moore BP, Brown WV, Rothschild M (1990) Methylalkylpyrazines in aposematic insects, their host plants and mimics. Chemoecology 1:43. https://doi.org/10.1007/BF01325227
Nyffeler M, Birkhofer K (2017) An estimated 400–800 million tons of prey are annually killed by the global spider community. Die Naturwissenschaften 104(3–4):30
Orr AG, Trigo JR, Witte L, Hartmann T (1996) Sequestration of pyrrolizidine alkaloids by larvae of Tellervo zoilus (Lepidoptera: Ithomiinae) and their role in the chemical protection of adults against the spider Nephila maculata (Araneidae). Chemoecology 7:68–73
Pekár S, Brabec M (2018) Generalized estimating equations: a pragmatic and flexible approach to the marginal GLM modelling of correlated data in the behavioural sciences. Ethology 124:86–93. https://doi.org/10.1111/eth.12713
Petschenka G, Bramer C, Pankoke H, Dobler S (2011) Evidence for a deterrent effect of cardenolides on Nephila spiders. Basic Appl Ecol 12:260–267
Priebusch P (2012) Auswirkungen von Schreckstoffen in Marienkäfern auf das Fressverhalten von Brückenspinnen. BSc Thesis, Fachbereich Biologie, Universität Hamburg
R Development Core Team (2008) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. ISBN 3-900051-07-0. https://www.R-project.org.
Rojas B, Burdfield-Steel E, Pakkanen H, Suisto K, Maczka M, Schulz S, Mappes J (2017) How to fight multiple enemies: target-specific chemical defences in an aposematic moth. Proc R Soc B 284:20171424
Rojas B, Mappes J, Burdfield-Steel E (2019) Multiple modalities in insect warning displays have additive effects against wild avian predators. Behav Ecol Sociobiol 73:37. https://doi.org/10.1007/s00265-019-2643-6
Rothschild M, Moore BP, Brown WV (1984) Pyrazines as warning odour components in the Monarch butterfly, Danaus plexippus, and in moths of the genera Zygaena and Amata (Lepidoptera). Biol J Linn Soc 23:375–380
Rowe C, Guilford T (1996) Hidden colour aversions in domestic chicks triggered by pyrazine odours of insect warning displays. Nature 383:520–522. https://doi.org/10.1038/383520a0
Sloggett JJ (2010) Predation of ladybird beetles by the orb-web spider Araneus diadematus. Biocontrol 55:631–638. https://doi.org/10.1007/s10526-010-9291-0
Speed MP, Ruxton GD, Mappes J, Sherratt TN (2012) Why are defensive toxins so variable? An evolutionary perspective. Biol Rev 87:874–884. https://doi.org/10.1111/j.1469-185X.2012.00228.x
Tan WH, Tao L, Hoang KM, Hunter MD, de Roode JC (2018) The effects of milkweed induced defense on parasite resistance in monarch butterflies Danaus Plexippus. J Chem Ecol 44(11):1040–1044. https://doi.org/10.1007/s10886-018-1007-4
Trigo JR, Brown KS Jr, Witte L, Hartmann T, Ernst L, Barata LES (1996) Pyrrolizidine alkaloids: different acquisition and use patterns in Apocynaceae and Solanaceae feeding ithomiine butterflies (Lepidoptera: Nymphalidae). Bot J Linn Soc 58:99–123
Vasconcellos-Neto J, Lewinsohn TM (1984) Discrimination and release of unpalatable butterflies by Nephila clavipes, a neotropical orb-weaving spider. Ecol Entomol 9:337–344
Zvereva EL, Kozlov MV (2016) The costs and effectiveness of chemical defenses in herbivorous insects: a meta-analysis. Ecol Monogr 86:107–124. https://doi.org/10.1890/15-0911.1
Acknowledgments
Thanks go to all the technicians in the spider lab at Universität Hamburg for breeding and maintaining the spiders used, Kaisa Suisto and the greenhouse workers at the University of Jyväskylä for rearing the moths, and two anonymous referees for comments on an earlier version of this manuscript. We are particularly grateful to Nadja Rathjen who carried out experiment 3. EBS was funded by the Centre of Excellence in Biological Interactions, via the Academy of Finland (Project No. 252411). The exchange between Universität Hamburg and the University of Jyväskylä was funded by the Landesforschungsförderung Hamburg, LFF OS 16-2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Günther Raspotnig.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Burdfield-Steel, E.R., Schneider, J.M., Mappes, J. et al. Testing the effectiveness of pyrazine defences against spiders. Chemoecology 30, 139–146 (2020). https://doi.org/10.1007/s00049-020-00305-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00049-020-00305-5