Abstract
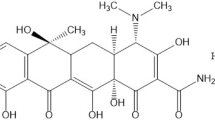
The aim of this work was to prepare hybrid organo-layered materials using intercalation process then evaluated them as a drug release and antibacterial materials. Four kinds of organo-layered materials cetyltrimethylammonium bromide-bentonite (CTA-bent), ampicillin-bentonite (AMP-bent), CTA-magadiite (CTA-mag) and ampicillin-magadiite (AMP-mag) have been prepared and characterized using several techniques. The results showed that the CTA and AMP molecules are incorporated between the interlayer spaces, subsequently leading to an increase of the basal spacing of bentonite and magadiite solids, in which preserving their two-dimensional character. Antibacterial activities of the intercalated layered materials were determined against Gram positive and Gram negative bacteria. The ranking of antibacterial activity of the intercalated materials was as organo-modified bentonite > organo-modified magadiite. In vitro drug release profile in simulated intestinal fluid pH 7.4 at 37 °C was evaluated. A satisfactory percent of cumulative AMP release from AMP-bent was observed. The cumulative drug release from AMP-bent and AMP-mag was 31.13% and 25.24%, respectively. This work highlighted the role of the natural surface of layered materials in the release of a drug by involving surface-drug interaction forces.








Similar content being viewed by others
References
Lazzara, G., Cavallaro, G., Panchal, A., Fakhrullin, R., Stavitskaya, A., Vinokurov, V., Lvov, Y.: An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 35, 42–50 (2018)
García-Villén, F., Carazo, E., Borrego-Sánchez, A., Sánchez-Espejo, R., Cerezo, P., Viseras, C., Aguzzi, C.: in Modified Clay and Zeolite Nanocomposite Materials, pp. 129–166. Elsevier, Amsterdam (2019)
Nabipour, H.: Design and evaluation of non-steroidal anti-inflammatory drug intercalated into layered zinc hydroxide as a drug delivery system. J. Inorg. Organomet. Polym. Mater. 29(5), 1807–1817 (2019)
Aguzzi, C., Cerezo, P., Viseras, C., Caramella, C.: Use of clays as drug delivery systems: possibilities and limitations. Appl. Clay Sci. 36, 22–36 (2007)
Rives, V., del Arco, M., Martín, C.: Intercalation of drugs in layered double hydroxides and their controlled release: a review. Appl. Clay Sci. 88, 239–269 (2014)
Cheikh, D., García-Villén, F., Majdoub, H., Zayani, M.B., Viseras, C.: Complex of chitosan pectin and clay as diclofenac carrier. Appl. Clay Sci. 172, 155–164 (2019)
Roth, W.J., Gil, B., Makowski, W., Marszalek, B., Eliášová, P.: Layer like porous materials with hierarchical structure. Chem. Soc. Rev. 45, 3400–3438 (2016)
Rebitski, E.P., Souza, G.P., Santana, S.A., Pergher, S.B., Alcântara, A.C.: Bionanocomposites based on cationic and anionic layered clays as controlled release devices of amoxicillin. Appl. Clay Sci. 173, 35–45 (2019)
Gao, J., Feng, Y., Guo, W., Jiang, L.: Nanofluidics in two-dimensional layered materials: inspirations from nature. Chem. Soc. Rev. 46, 5400–5424 (2017)
Selvam, T., Inayat, A., Schwieger, W.: Reactivity and applications of layered silicates and layered double hydroxides. Dalton Trans. 43, 10365–10387 (2014)
Zheng, J., Luan, L., Wang, H., Xi, L., Yao, K.: Study on ibuprofen/montmorillonite intercalation composites as drug release system. Appl. Clay Sci. 36, 297–301 (2007)
Joshi, G.V., Kevadiya, B.D., Patel, H.A., Bajaj, H.C., Jasra, R.V.: Montmorillonite as a drug delivery system: intercalation and in vitro release of timolol maleate. Int. J. Pharm. 374, 53–57 (2009)
Nunes, A.R., Araújo, K.R., Moura, A.O., Prado, A.G.: Magadiite as a support for the controlled release of herbicides. Chem. Pap. 72, 479–486 (2018)
Ghadiri, M., Chrzanowski, W., Rohanizadeh, R.: Biomedical applications of cationic clay minerals. RSC Adv. 5, 29467–29481 (2015)
Jafarbeglou, M., Abdouss, M., Shoushtari, A.M., Jafarbeglou, M.: Clay nanocomposites as engineered drug delivery systems. RSC Adv. 6, 50002–50016 (2016)
Khlibsuwan, R., Siepmann, F., Siepmann, J., Pongjanyakul, T.: Chitosan-clay nanocomposite microparticles for controlled drug delivery: Effects of the MAS content and TPP crosslinking. J. Drug Deliv. Sci. Technol. 40, 1–10 (2017)
Abdelkrim, S., Mokhtar, A., Djelad, A., Bennabi, F., Souna, A., Bengueddach, A., Sassi, M.: Chitosan/Ag-bentonite nanocomposites: preparation, characterization, swelling and biological properties. J. Inorg. Organomet. Polym. Mater. (2019). https://doi.org/10.1007/s10904-019-01219-8
Mokhtar, A., Abdelkrim, S., Djelad, A., Sardi, A., Boukoussa, B., Sassi, M., Bengueddach, A.: Adsorption behavior of cationic and anionic dyes on magadiite-chitosan composite beads. Carbohydr. Polym. 229, 115399 (2020)
Zhao, J., Zhang, Y., Zhang, S., Wang, Q., Chen, M., Hu, T., Meng, C.: Synthesis and characterization of Mn-Silicalite-1 by the hydrothermal conversion of Mn-magadiite under the neutral condition and its catalytic performance on selective oxidation of styrene. Microporous Mesoporous Mater. 268, 16–24 (2018)
Joseph, A., Vellayan, K., González, B., Vicente, M.A., Gil, A.: Effective degradation of methylene blue in aqueous solution using Pd-supported Cu-doped Ti-pillared montmorillonite catalyst. Appl. Clay Sci. 168, 7–10 (2019)
Nunes, A.R., Moura, A.O., Prado, A.G.: Calorimetric aspects of adsorption of pesticides 2, 4-D, diuron and atrazine on a magadiite surface. J. Therm. Anal. Calorim. 106, 445–452 (2011)
Joshi, G.V., Patel, H.A., Kevadiya, B.D., Bajaj, H.C.: Montmorillonite intercalated with vitamin B1 as drug carrier. Appl. Clay Sci. 45, 248–253 (2009)
Sun, B., Zhang, M., Zhou, N., Chu, X., Yuan, P., Chi, C., Wu, F., Shen, J.: Study on montmorillonite–chlorhexidine acetate–terbinafine hydrochloride intercalation composites as drug release systems. RSC Adv. 8, 21369–21377 (2018)
Vieira, R.B., Moura, P.A., Vilarrasa-García, E., Azevedo, D.C., Pastore, H.O.: Pastore, polyamine-grafted magadiite: high CO2 selectivity at capture from CO2/N2 and CO2/CH4 mixtures. J. Util. 23, 29–41 (2018)
Cavalcanti, G.R., Fonseca, M.G., da Silva Filho, E.C., Jaber, M.: Thiabendazole/bentonites hybrids as controlled release systems. Colloids Surf. B 176, 249–255 (2019)
Andrade, K.N., Pérez, A.M.P., Arízaga, G.G.C.: Passive and active targeting strategies in hybrid layered double hydroxides nanoparticles for tumor bioimaging and therapy. Appl. Clay Sci. 181, 105214 (2019)
Yahia, Y., García-Villén, F., Djelad, A., Belaroui, L.S., Sanchez-Espejo, R., Sassi, M., López-Galindo, A., Viseras, C.: Crosslinked palygorskite-chitosan beads as diclofenac carriers. Appl. Clay Sci. 180, 105169 (2019)
Almasy, L., Putz, A.-M., Tian, Q., Kopitsa, G.P., Khamova, T.V., Barabas, R., Rigo, M., Bota, A., Wacha, A., Mirica, M.: Hybrid mesoporous silica with controlled drug release. J. Serbian Chem. Soc. 84, 1027–1039 (2019)
Buffet-Bataillon, S., Tattevin, P., Bonnaure-Mallet, M., Jolivet-Gougeon, A.: Emergence of resistance to antibacterial agents: the role of quaternary ammonium compounds—a critical review. Int. J. Antimicrob. Agents 39, 381–389 (2012)
Birnie, C.R., Malamud, D., Schnaare, R.L.: Antimicrobial evaluation of N-alkyl betaines and N-alkyl-N, N-dimethylamine oxides with variations in chain length. Antimicrob. Agents Chemother. 44, 2514–2517 (2000)
Peng, S., Gao, Q., Du, Z., Shi, J.: Precursors of TAA-magadiite nanocomposites. Appl. Clay Sci. 31, 229–237 (2006)
Xue, W., He, H., Zhu, J., Yuan, P.: FTIR investigation of CTAB–Al–montmorillonite complexes. Spectrochim Acta Part A 67, 1030–1036 (2007)
Park, C.W., Kim, B.H., Yang, H.-M., Seo, B.-K., Moon, J.-K., Lee, K.-W.: Removal of cesium ions from clays by cationic surfactant intercalation. Chemosphere 168, 1068–1074 (2017)
Sassi, M., Miehé-Brendlé, J., Patarin, J., Bengueddach, A., De Gruyter.: Na-magadiite prepared in a water/alcohol medium: synthesis, characterization and use as a host material to prepare alkyltrimethylammonium-and Si-pillared derivates. J. Clay. Miner. 40, 369–378 (2005)
Mokhtar, A., Djelad, A., Adjdir, M., Zahraoui, M., Bengueddach, A., Sassi, M.: Intercalation of hydrophilic antibiotic into the interlayer space of the layered silicate magadiite. J. Mol. Struct. 1171, 190–195 (2018)
Dizman, B., Badger, J.C., Elasri, M.O., Mathias, L.J.: Antibacterial fluoromicas: a novel delivery medium. Appl. Clay Sci. 38, 57–63 (2007)
Trikeriotis, M., Ghanotakis, D.F.: Intercalation of hydrophilic and hydrophobic antibiotics in layered double hydroxides. Int. J. Pharm. 332, 176–184 (2007)
Waworuntu, G., Hanelin, G.A., Wiyanto, L.D., Laysandra, L., Soetaredjo, F.E.: Preparation of antibacterial bentonite β lactam antibiotic composite. EQA Environ. Qual. 32, 45–55 (2019)
Parolo, M., Avena, M., Pettinari, G., Zajonkovsky, I., Valles, J., Baschini, M.: Antimicrobial properties of tetracycline and minocycline-montmorillonites. Appl. Clay Sci. 49, 194–199 (2010)
Wang, C.-J., Li, Z., Jiang, W.-T., Jean, J.-S., Liu, C.-C.: Cation exchange interaction between antibiotic ciprofloxacin and montmorillonite. J. Hazard. Mater. 183, 309–314 (2010)
Chung, H.-E., Park, D.-H., Choy, J.-H., Choi, S.-J.: Intracellular trafficking pathway of layered double hydroxide nanoparticles in human cells: size-dependent cellular delivery. Appl. Clay Sci. 65, 24–30 (2012)
Yang, J.-H., Han, Y.-S., Park, M., Park, T., Hwang, S.-J., Choy, J.-H.: New inorganic-based drug delivery system of indole-3-acetic acid-layered metal hydroxide nanohybrids with controlled release rate. Chem. Mater. 19, 2679–2685 (2007)
Oh, J.-M., Park, D.-H., Choi, S.-J., Choy, J.-H.: LDH nanocontainers as bio-reservoirs and drug delivery carriers. Recent Pat. Nanotechnol. 6, 200–217 (2012)
Cherifi, Z., Boukoussa, B., Zaoui, A., Belbachir, M., Meghabar, R.: Structural, morphological and thermal properties of nanocomposites poly (GMA)/clay prepared by ultrasound and in-situ polymerization. Ultrason. Sonochem. 48, 188–198 (2018)
Kahr, G., Madsen, F.: Determination of the cation exchange capacity and the surface area of bentonite, illite and kaolinite by methylene blue adsorption. Appl. Clay Sci. 9, 327–336 (1995)
Mokhtar, A., Djelad, A., Bengueddach, A., Sassi, M.: CuNPs-magadiite/chitosan nanocomposite beads as advanced antibacterial agent: synthetic path and characterization. Int. J. Biol. Macromol. 118, 2149–2155 (2018)
Mokhtar, A., Djelad, A., Bengueddach, A., Sassi, M.: Structural and antibacterial properties of HyZnxNa2-xSi14O29nH2O layered silicate compounds, prepared by ion-exchange reaction. J. Inorg. Organomet. Polym. Mater. 29(3), 1029–1038 (2019)
Zahraoui, M., Mokhtar, A., Adjdir, M., Bennabi, F., Khaled, R., Djelad, A., Bengueddach, A., Sassi, M.: Preparation of Al-magadiite material, copper ions exchange and effect of counter-ions: antibacterial and antifungal applications. Res. Chem. Intermed. 45(2), 633–644 (2018)
Galindo, A.L., Ruiz, J.T., Lopez, J.G.: Mineral quantification in sepiolite-palygorskite deposits using X-ray diffraction and chemical data. Clay Miner. 31, 217–224 (1996)
Zhirong, L., Uddin, M.A., Zhanxue, S.: FT-IR and XRD analysis of natural Na-bentonite and Cu (II)-loaded Na-bentonite. Spectrochim. Acta Part A 79, 1013–1016 (2011)
Zahaf, F., Dali, N., Marouf, R., Ouadjenia, F., Schott, J.: Application of hydroxy-aluminum-and cetyltrimethylammonium bromide-intercalated bentonite for removing acid and reactive dyes. Desalin. Water Treat. 57, 21045–21053 (2016)
Abas SNA, Ismail MHS, Siajam SI, Kamal ML, Comparative study on adsorption of Pb (II) ions by alginate beads and mangrove-alginate composite beads. In A. Hadi, F. Hamzah and M.N.M. Rodhi (eds) Advanced Materials Research. Trans Tech Publications. 1113, (2015)
Brindley, G.: Unit cell of magadiite in air, in vacuo, and under other conditions. Am. Mineral. 54, 1583–1591 (1969)
Schwieger, W., Lagaly, G., Auerbach, S., Carrado, K., Dutta, P.: Marcel Dekker, Inc., New York, pp. 541–551 (2004)
Mokhtar, A., Djelad, A., Bengueddach, A., Sassi, M.: Biopolymer-layered polysilicate micro/nanocomposite based on chitosan intercalated in magadiite. Res. Chem. Intermed. 44(11), 6469–6478 (2018)
Boudahri, M., Bouazza, D., Adjdir, M., Miloudi, H., Abdelkader, N., Tayeb, A.: Remediation of copper ions from aqueous solution using hybrid magadiite: kinetics, isotherm and mechanism of removal. Res. Chem. Intermed. 44, 6105–6117 (2018)
Shirzad-Siboni, M., Khataee, A., Hassani, A., Karaca, S.: Preparation, characterization and application of a CTAB-modified nanoclay for the adsorption of an herbicide from aqueous solutions: kinetic and equilibrium studies. C.R. Chim. 18, 204–214 (2015)
Benkhatou, S., Djelad, A., Sassi, M., Bouchekara, M., Bengueddach, A.: Lead(II) removal from aqueous solutions by organic thiourea derivatives intercalated magadiite. Desalin. Water Treat. 57, 9383–9395 (2016)
Paluszkiewicz, C., Holtzer, M., Bobrowski, A.: FTIR analysis of bentonite in moulding sands. J. Mol. Struct. 880, 109–114 (2008)
Alabarse, F.G., Conceição, R.V., Balzaretti, N.M., Schenato, F., Xavier, A.M.: In-situ FTIR analyses of bentonite under high-pressure. Appl. Clay Sci. 51, 202–208 (2011)
Wang, L., Wang, A.: Adsorption properties of Congo Red from aqueous solution onto surfactant-modified montmorillonite. J. Hazard. Mater. 160, 173–180 (2008)
Firyal, M., Rahi, F.A., Wessal, M.K., Alshather, A.I., Shurooq, S.: Modification of starch with allopurinol and ampicilline as sulfonamide derivatives. Al-Nahrain J. Sci. 17, 21–26 (2014)
Rojo, J.M., Ruiz-Hitzky, E., Sanz, J.: Proton-sodium exchange in magadiite. Spectroscopic study (NMR, IR) of the evolution of interlayer OH groups. Inorg. Chem. 27, 2785–2790 (1988)
Li, S., Mao, Y., Ploehn, H.J.: Interlayer functionalization of magadiite with sulfur-containing organosilanes. Colloids Surf. A 506, 320–330 (2016)
Taleb, K., Pillin, I., Grohens, Y., Saidi-Besbes, S.: Gemini surfactant modified clays: effect of surfactant loading and spacer length. Appl. Clay Sci. 161, 48–56 (2018)
Kooli, F., Liu, Y., Abboudi, M., Rakass, S., Hassani, H., Ibrahim, S., Al-Faze, R.: Application of organo-magadiites for the removal of eosin dye from aqueous solutions: thermal treatment and regeneration. Molecules 23, 2280 (2018)
Attar, K., Bouazza, D., Miloudi, H., Tayeb, A., Boos, A., Sastre, A.M., Demey, H.: Cadmium removal by a low-cost magadiite-based material: characterization and sorption applications. J. Environ. Chem. Eng. 6, 5351–5360 (2018)
Kooli, F., Mianhui, L., Alshahateet, S.F., Chen, F., Yinghuai, Z.: Characterization and thermal stability properties of intercalated Na-magadiite with cetyltrimethylammonium (C16TMA) surfactants. J. Phys. Chem. Solids 67, 926–931 (2006)
Schwieger, W., Selvam, T., Gravenhorst, O., Pfänder, N., Schlögl, R., Mabande, G.: Intercalation of [Pt (NH3)4]2+ ions into layered sodium silicate magadiite: a useful method to enhance their stabilisation in a highly dispersed state. J. Phys. Chem. Solids 65, 413–420 (2004)
Mokhtar, A., Medjhouda, Z.A.K., Djelad, A., Boudia, A., Bengueddach, A., Sassi, M.: Structure and intercalation behavior of copper II on the layered sodium silicate magadiite material. Chem. Pap. 72, 39–50 (2018)
Holešová, S., Valášková, M., Plevová, E., Pazdziora, E., Matějová, K.: Preparation of novel organovermiculites with antibacterial activity using chlorhexidine diacetate. J. Colloid Interface Sci. 342, 593–597 (2010)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mokhtar, A., Bennabi, F., Abdelkrim, S. et al. Evaluation of intercalated layered materials as an antimicrobial and drug delivery system: a comparative study. J Incl Phenom Macrocycl Chem 96, 353–364 (2020). https://doi.org/10.1007/s10847-020-00978-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-020-00978-z