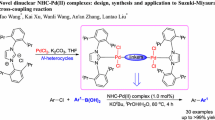
Abstract
A new air and moisture stable PEPPSI (PEPPSI: pyridine-enhanced pre-catalyst preparation, stabilisation, and initiation) themed palladium N-heterocyclic carbene (NHC) complex [Pd(L)Br2(Py)] (1) [L: 2-flurobenzyl)-1-(4-methoxyphenyl)-1H-imidazolline-2-ylidene] was synthesized and characterized. The structure of complex 1 was determined by X-ray single-crystal analysis. The palladium center in 1 adopted a square planar geometry with carbene and pyridine ligands occupying the mutual trans position. The complex 1 was employed to catalyze the Mizoroki-Heck cross-coupling reactions of aryl bromides/iodides with styrene in water. To the best of our knowledge, this is the first report where a Pd-PEPPSI catalyst was successfully employed in aqueous-phase Mizoroki-Heck reaction. Good to excellent yields of cross-coupling products were obtained with a range of representative aryl bromides/iodides under relatively mild conditions (100 °C, 1 mol% of 1).
Graphic abstract
A new palladium PEPPSI complex was synthesized, characterized and evaluated as catalyst for the Mizoroki-Heck reaction in aqueous medium. Both electron-donating and electron-withdrawing aryl halides (bromides and iodides) reacted with styrene to give corresponding coupling products in moderate to excellent yields.







Similar content being viewed by others
References
Selected examples: (a) Hsu Y C, Shen J S, Lin B C, Chen W C, Chan Y T, Ching W M, Yap G P A, Hsu C P and Ong T G 2015 Synthesis and Isolation of an Acyclic Tridentate Bis(pyridine) carbodicarbene and Studies on Its Structural Implications and Reactivities Angew. Chem. Int. Ed. 54 2420; (b) Gonzalez S D, Marion N and Nolan S. P 2009 N-Heterocyclic Carbenes in Late Transition Metal Catalysis Chem. Rev. 109 3612; (c) Schaper L A, Hock S J, Herrmann W A and Kühn F E 2013 Synthesis and Application of Water‐Soluble NHC Transition‐Metal Complexes Angew. Chem. Int. Ed. 52 270; (d) Hopkinson M N, Richter C, Schedler M and Glorius F 2014 An overview of N-heterocyclic carbenes Nature 510 485; (e) Gurbuz N, Karaca E O, Ozdemir I and Cetinkaya B 2015 Cross coupling reactions catalyzed by (NHC)Pd(II) complexes Turk. J. Chem. 39 1115
Selected examples: (a) Lu D D, He X X and Liu F S 2017 Bulky Yet Flexible Pd-PEPPSI-IPentAn for the Synthesis of Sterically Hindered Biaryls in Air J. Org. Chem. 82 10898; (b) Meiries S, Duc G L, Chartoire A, Collado A, Speck K, Arachchige K S A, Slawin A M Z and Nolan S P 2013 Large yet Flexible N-Heterocyclic Carbene Ligands for Palladium Catalysis Chem. Eur. J. 19 17358; (c) Lan X B, Li Y, Li Y F, Shen D S, Ke Z and Liu F S 2017 Flexible Steric Bulky Bis(Imino)acenaphthene (BIAN)-Supported N-Heterocyclic Carbene Palladium Precatalysts: Catalytic Application in Buchwald–Hartwig Amination in Air J. Org. Chem. 82 2914; (d) Zeiler A, Rudolph M, Rominger F and Hashmi A S K 2015 An Alternative Approach to PEPPSI Catalysts: From Palladium Isonitriles to Highly Active Unsymmetrically Substituted PEPPSI Catalysts Chem. Eur. J. 21 11065; (e) Akkoc S, Ilhan I O, Gok Y, Kayser V 2017 Carbon-carbon bond formation catalyzed by PEPPSI Pd-NHC Inorg. Chim. Acta 461 52
Balinge K R and Bhagat P R 2017 Palladium–N-heterocyclic carbene complexes for the Mizoroki–Heck reaction: An appraisal C. R. Chimie. 20 773
Ortiz A, Sal P G, Flores J C and Jesus E de 2018 Highly Recoverable Pd(II) Catalysts for the Mizoroki–Heck Reaction Based on N-Heterocyclic Carbenes and Poly(benzyl ether) Dendrons Organometallics 37 3598
Ma M T and Lu J M 2012 Dinuclear Pd(II)–NHC complex derived from proline and its application toward Mizoroki–Heck reaction performed in water Appl. Organomet. Chem. 26 175
Herrmann W A, Bohm V P W, Gstottmayr C W K, Grosche M, Reisinger C P and Weskamp T 2001 Synthesis, structure and catalytic application of palladium(II) complexes bearing N-heterocyclic carbenes and phosphines J. Organomet. Chem. 617 616
(a) Yuan D, Teng Q and Huynh H V 2014 Template-Directed Synthesis of Palladium(II) Sulfonate-NHC Complexes and Catalytic Studies in Aqueous Mizoroki−Heck Reactions Organometallics 33 1794; (b) Gülcemal S, Kahraman S, Daran J C, Çetinkaya E and Çetinkaya B 2009 The synthesis of oligoether-substituted benzimidazolium bromides and their use as ligand precursors for the Pd-catalyzed Heck coupling in water J. Organomet. Chem. 694 3580; (c) Scho¨nfelder D, Nuyken O and Weberskirch R 2005 Heck and Suzuki coupling reactions in water using poly(2-oxazoline)s functionalized with palladium carbene complexes as soluble, amphiphilic polymer supports J. Organomet. Chem. 690 3580; (d) Scho1nfelder D, Fischer K, Schmidt M, Nuyken O and Weberskirch R 2005 Poly(2-oxazoline)s Functionalized with Palladium Carbene Complexes: Soluble, Amphiphilic Polymer Supports for C-C Coupling Reactions in Water Macromolecules 38 254; (e) Özdemir I, Gürbüz N, Gök Y and Çetinkaya B 2008 N‐functionalized azolin‐2‐ylidene‐palladium‐catalyzed Heck reaction Heteroatom Chem. 19 82
Selected examples: (a) O’Brien C J, Kantchev E A, Valente B C, Hadei N, Chass G A, Lough A, Hopkinson A C and Organ M G 2006 Easily Prepared Air- and Moisture Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction Chem. Eur. J. 12 4743; (b) Organ M G, Hadi M A, Avola S, Hadei N, Nasielski J, O’Brien C J and Valente C 2006 Biaryls Made Easy: PEPPSI and the Kumada–Tamao–Corriu Reaction Chem. Eur. J. 13 150; (c) Organ M G, Calimsiz S, Sayah M, Hoi K and Lough A J 2009 Pd-PEPPSI-IPent: An Active, Sterically Demanding Cross-Coupling Catalyst and Its Application in the Synthesis of Tetra-Ortho-Substituted Biaryls Angew. Chem. Int. Ed. 48 2383; (d) Çalimsiz S, Sayah M, Mallik D and Organ M G 2010 Pd-PEPPSI-IPent: Low-Temperature Negishi Cross-Coupling for the Preparation of Highly Functionalized, Tetra-ortho-Substituted Biaryls Angew. Chem. Int. Ed. 49 2014; (e) Hoi K H, Calimsiz S, Froese R D J, Hopkinson A C and Organ M G 2012 Amination with Pd-NHC Complexes: Rate and Computational Studies Involving Substituted Aniline Substrates Chem. Eur. J. 18 145
(a) Lan X B, Chen F M, Ma B B, Shen D S and Liu F S 2016 Pd-PEPPSI Complexes Bearing Bulky [(1,2-Di-(tert-butyl)acenaphthyl] (DtBu-An) on N-Heterocarbene Backbones: Highly Efficient for Suzuki–Miyaura Cross-Coupling under Aerobic Conditions Organometallics 35 3852; (b) Touj N, Gurbuz N, Hamdi N, Yaşa Sand Ozdemir I 2018 Palladium PEPPSI complexes: Synthesis and catalytic activity on the Suzuki-Miyaura coupling reactions for aryl bromides at room temperature in aqueous media Inorg. Chim. Acta 478 187; (c) Lei P, Meng G, Ling Y, An J and Szostak M 2017 Pd-PEPPSI: Pd-NHC Precatalyst for Suzuki–Miyaura Cross-Coupling Reactions of Amides J. Org. Chem. 82 6638; (d) Steeples E, Kelling A, Schilde U and Esposito D 2016 Amino acid-derived N-heterocyclic carbene palladium complexes for aqueous phase Suzuki–Miyaura couplings New J. Chem. 40 4922; (e) Nava D R, Hernandez A A, Rheingold A L, Castillo O R S and Espinos D M 2019 Hydroxyl-functionalized triazolylidene-based PEPPSI complexes: metallacycle formation effect on the Suzuki coupling reaction Dalton Trans. 48 3214; (f) Imik F,Yaşar S and Ozdemir I 2019 Synthesis and investigation of catalytic activity of phenylene –And biphenylene bridged bimetallic Palladium-PEPPSI complexes J. Organomet. Chem. 896 162; (g) Kaloglu N and Ozdemir I 2019 PEPPSI-Pd-NHC catalyzed Suzuki-Miyaura cross-coupling reactions in aqueous media Tetrahedron 75 2306; (h) Sikorski W, Zawartka W and Trzeciak A M 2018 PEPPSI-type complexes with small NHC ligands obtained according to the new method efficiently catalyzed Suzuki-Miyaura reaction J. Organomet. Chem. 867 323; (i) Mondal M, Joji J and Choudhury J 2018 Coordination-polymer anchored single-site ‘Pd-NHC’ catalyst for Suzuki-Miyaura coupling in water J. Chem. Sci. 130 83
(a) Zhang Y, Lavigne G, Lugan N and Cesar V 2017 Buttressing Effect as a Key Design Principle towards Highly Efficient Palladium/N-Heterocyclic Carbene Buchwald–Hartwig Amination Catalysts Chem. Eur. J. 23 13792; (b) Sharif S, Rucker R P, Chandrasoma N, Mitchell D, Rodriguez M J, Froese R D J and Organ M G 2015 Selective Monoarylation of Primary Amines Using the Pd-PEPPSI-IPentCl Precatalyst Angew. Chem. Int. Ed. 54 9507; (c) Yang J 2017 Heteroleptic (N-heterocyclic carbene)–Pd–pyrazole (indazole) complexes: Synthesis, characterization and catalytic activities towards C–C and C–N cross-coupling reactions Appl. Organometal. Chem. 31 3734
(a) Dash C, Shaikh M M and Ghosh P 2009 Fluoride-Free Hiyama and Copper and Amine-Free Sonogashira Coupling in Air in a Mixed Aqueous Medium by a Series of PEPPSI-Themed Precatalysts Eur. J. Inorg. Chem. 1608; (b) Gallop C W D, Chen M T and Navarro O 2014 Sonogashira Couplings Catalyzed by Collaborative (N-Heterocyclic Carbene)-Copper and-Palladium Complexes Org. Lett. 16 3724; (c) Boubakri L, Yasar S, Dorcet V, Roisnel T, Bruneau C, Hamdib N and Ozdemir I 2017 Synthesis and catalytic applications of palladium N-heterocyclic carbene complexes as efficient pre-catalysts for Suzuki–Miyaura and Sonogashira coupling reactions New J. Chem. 41 5105
(a) Panyam P K R, Ugale B and Gandhi T 2018 Palladium(II)/N-Heterocyclic Carbene Catalyzed One-Pot Sequential α-Arylation/Alkylation: Access to 3,3-Disubstituted Oxindoles J. Org. Chem. 83 7622; (b) Kaloglu N, Kaloglu M, Tahir M N, Arici C, Bruneau C, Doucet H, Dixneuf P H, Çetinkaya B and Ozdemir I 2018 Synthesis of N-heterocyclic carbene-palladium-PEPPSI complexes and their catalytic activity in the direct C-H bond activation J. Organomet. Chem. 867 404; (c) Aktaş A, Barut Celepci D and Gök Y 2019 Novel 2-hydroxyethyl substituted N-coordinate-Pd(II)(NHC) and bis(NHC)Pd(II) complexes: Synthesis, characterization and the catalytic activity in the direct arylation reaction J. Chem. Sci. 131 78
Valente C, Pompeo M, Sayah M and Organ M G 2014 Carbon–Heteroatom Coupling Using Pd-PEPPSI Complexes Org. Proc. Res. Dev. 18 180
(a) Lu H, Wang L, Yang F, Wua R and Shen W 2014 Cross-coupling reactions catalyzed by an N-heterocyclic carbene–Pd(II) complex under aerobic and CuI-free conditions RSC Adv. 4 30447; (b) Lin Y C, Hsueh H H, Kanne S, Chang L K, Liu F C and Lin I J B 2013 Efficient PEPPSI-Themed Palladium N-Heterocyclic Carbene Precatalysts for the Mizoroki–Heck Reaction Organometallics 32 3859; (c) Keske E C, Zenkina O V, Wang R and Crudden C M 2012 Synthesis and Structure of Palladium 1,2,3-Triazol-5-ylidene Mesoionic Carbene PEPPSI Complexes and Their Catalytic Applications in the Mizoroki-Heck Reaction Organometallics 31 6215
Akkoc M, Imik F, Yasar S, Dorcet V, Roisnel T, Bruneau C and Ozdemir I 2017 An Efficient Protocol for Palladium N-Heterocyclic Carbene-Catalysed Suzuki-Miyaura Reaction at room temperature ChemistrySelect 2 5729
SAINT Plus (v 6.14) Bruker AXS Inc., Madison, WI, 2008
Bruker AXS Inc., Madison, WI, 2008
Sheldrick G M 1997 SHELXS and SHELXL97 Programs for structure refinement and solution, Göttingen, Germany
Spek A L 2002 PLATON, A Multipurpose Crystallographic Tool Utrecht University, Utrecht, Netherland
Spek A L 2003 Single-crystal structure validation with the program PLATON J. Appl. Cryst. 36 7
Roy B, Bar A K, Gole B and Mukherjee P S 2013 Fluorescent Tris-Imidazolium Sensors for Picric Acid Explosive J. Org. Chem. 78 1306
Jahnke M C, Hussain M, Hupka F, Pape T, Ali S, Hahn F E and Cavell K J 2009 Synthesis of pyrazine-bridged diimidazolium salts and their application in palladium catalyzed Heck-type coupling reactions Tetrahedron 65 909
Lord P A, Noll B C, Olmstead M M and Balch A L 2001 A Remarkable Skeletal Rearrangement of a Coordinated Tetrapyrrole: Chemical Consequences of Palladium π-Coordination to a Bilindione J. Am. Chem. Soc. 123 10554
Viossat P B, Dung N H and Robert F 1993 Structure du trans-dichlorobis(pyridine)palladium(II) Acta Cryst. Sect. C C49 84
Lan X B, Li Y, Li Y F, Shen D S, Ke Z and Liu F S 2017 Flexible Steric Bulky Bis(Imino)acenaphthene (BIAN)-Supported N-Heterocyclic Carbene Palladium Precatalysts: Catalytic Application in Buchwald-Hartwig Amination in Air J. Org. Chem. 82 2914
Han Y, Huynh H V and Tan G K 2007 Syntheses and Characterizations of Pd(II) Complexes Incorporating a N-Heterocyclic Carbene and Aromatic N-Heterocycles Organometallics 26 6447
(a) Guest D, Silva V H M, Batista A P L, Roe S M, Braga A A C and Navarro O 2015 (N-Heterocyclic Carbene)-Palladate Complexes in Anionic Mizoroki–Heck Coupling Cycles: A Combined Experimental and Computational Study Organometallics 34 2463; (b) Schroeter F, Strassner T 2018 Understanding Anionic “Ligandless” Palladium Species in the Mizoroki–Heck Reaction Inorg. Chem. 57 5159
(a) Jadhav S N and Rode C V 2017 An efficient palladium catalyzed Mizoroki–Heck cross-coupling in water Green Chem. 19 5958; (b) Christoffel F and Ward T R 2018 Palladium-Catalyzed Heck Cross-Coupling Reactions in Water: A Comprehensive Review Catal. Lett. 148 489
Khazipov O V, Shevchenko M A, Chernenko A Y, Astakhov A V, Pasyukov D V, Eremin D B, Zubavichus Y V, Khrustalev V N, Chernyshev V M and Ananikov V P 2018 Fast and Slow Release of Catalytically Active Species in Metal/NHC Systems Induced by Aliphatic Amines Organometallics 37 1483
Acknowledgements
We gratefully acknowledge the financial support from the Department of Science and Technology, New Delhi (Grant no: CRG/2018/001669) UGC, New Delhi for SAP-DRS grant to the Department of Chemistry, Dibrugarh University. DB is thankful to UGC, NERO for providing a teacher’s fellowship under FIP programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Borah, D., Saha, B., Sarma, B. et al. A new PEPPSI type N-heterocyclic carbene palladium(II) complex and its efficiency as a catalyst for Mizoroki-Heck cross-coupling reactions in water. J Chem Sci 132, 51 (2020). https://doi.org/10.1007/s12039-020-1754-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-1754-y