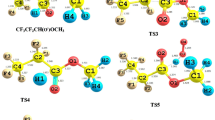
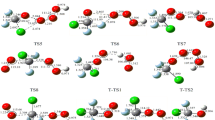
Abstract
The competition of hydrogen, halogen and pnicogen bonding to the stability of the atmospheric complexes is interesting, especially where the molecules by the most abundant greenhouse effect in the atmosphere are subject of interest. In the present work, we have computationally studied the addition of H2O to the NF3 and CF2Cl2 molecules to reveal the electronic and structural features of the NF3-H2O and CF2Cl2-H2O complexes through DFT, MP2 and CCSD (T) methods. The interaction energies, geometry and electronic properties including charge transfer, energy gap, NEDA and AIM analyses of all the complexes were calculated to discuss the nature and strength of intermolecular interactions. The results indicate that the role of halogen bonding is more obvious than that of hydrogen and pnicogen bonding, and compared with the NF3, CF2Cl2 is more effectively stabilized by the H2O molecules.
Graphic abstract
In the present work, we have studied the addition of H2O molecule to the NF3 and CF2Cl2 molecules to reveal the electronic and structural features of the NF3-H2O and CF2Cl2-H2O complexes through DFT, MP2 and CCSD (T) methods.





Similar content being viewed by others
References
Berger R, Resnati G, Metrangolo P, Weber E and Hulliger J 2011 Organic fluorine compounds: a great opportunity for enhanced materials properties Chem. Soc. Rev. 40 3496
Babudri F, Farinola G M, Naso F and Ragni R 2007 Fluorinated organic materials for electronic and optoelectronic applications: the role of the fluorine atom Chem. Commun. 1003
Jäckel C and Koksch B 2005 Fluorine in peptide design and protein engineering Eur. J. Org. Chem. 2005 4483
Zheng H, Comeforo K and Gao J 2008 Expanding the fluorous arsenal: tetrafluorinated phenylalanines for protein design J. Am. Chem. Soc. 131 18
Hughes R P 2010 Fluorine as a ligand substituent in organometallic chemistry: a second chance and a second research career J. Fluor. Chem. 131 1059
Thakur T S, Kirchner M T, Bläser D, Boese R and Desiraju G R 2010 C–H… F–C hydrogen bonding in 1, 2, 3, 5-tetrafluorobenzene and other fluoroaromatic compounds and the crystal structure of alloxan revisited CrystEngComm 12 2079
Scheiner S 2015 Noncovalent Forces (Springer: Berlin)
Grabowski S J 2006 Hydrogen Bonding: New Insights (Springer: Berlin)
Jeffrey G A and Saenger W 2012 Hydrogen Bonding in Biological Structures (Springer Science & Business Media: Berlin)
Thomas J C, Goronzy D P, Dragomiretskiy K, Zosso D, Gilles J, Osher S J, Bertozzi A L and Weiss P S 2016 Mapping buried hydrogen-bonding networks ACS Nano. 10 5446
Klinkhammer K W and Pyykko 1995 Ab initio interpretation of the closed-shell intermolecular E. cntdot.. cntdot.. cntdot. E attraction in dipnicogen (H2E-EH2)2 and dichalcogen (HE-EH)2 hydride model dimers Inorg. Chem. 34 4134
Sanz P, Yáñez M and Mó O 2002 Competition between X···H···Y intramolecular hydrogen bonds and X····Y (X = O, S, and Y = Se, Te) chalcogen–chalcogen interactions J. Phys. Chem. A 106 4661
Vila A, Vila E and Mosquera R A 2006 Topological characterisation of intermolecular lithium bonding Chem. Phys. 326 401
Politzer P, Murray J S and Concha M C 2008 σ-hole bonding between like atoms; a fallacy of atomic charges J. Mol. Model. 14 659
Meyer F and Dubois P 2013 Halogen bonding at work: recent applications in synthetic chemistry and materials science CrystEngComm 15 3058
Wilcken R, Zimmermann M O, Lange A, Joerger A C and Boeckler F M 2013 Principles and applications of halogen bonding in medicinal chemistry and chemical biology J. Med. Chem. 56 1363
Metrangolo P and Resnat G 2015 Halogen Bonding II (Springer: Berlin)
Politzer P, Murray J S and Clark T 2014 σ-Hole bonding: a physical interpretation In Halogen Bond. I (Springer: Berlin) p. 19
Arman H D 2008 Halogen bonding: fundamentals and applications (Springer Science & Business Media: Berlin)
Scheiner S 2013 Detailed comparison of the pnicogen bond with chalcogen, halogen, and hydrogen bonds Int. J. Quantum Chem. 113 1609
Müller K, Faeh C and Diederich F 2007 Fluorine in pharmaceuticals: looking beyond intuition Science 317 1881
Wang J, Sánchez-Roselló M, Aceña J L, del Pozo C, Sorochinsky A E, Fustero S, Soloshonok V A and Liu H 2013 Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011) Chem. Rev. 114 2432
Scheele R, McNamara B, Casella A M and Kozelisky A 2012 On the use of thermal NF3 as the fluorination and oxidation agent in treatment of used nuclear fuels J. Nucl. Mater. 424 224
Merlet P and Fluorine F 2013 Compounds with Oxygen and Nitrogen (Springer Science & Business Media: Berlin)
Tyagi A and Chatterjee D S 2013 Liquid crystal display: environment & technology Int. J. Environ. Eng. Sci. Technol. Res. 1 2
Tsakiris V, Kappel W and Alecu G 2011 Solid state diffusion welding of Cu-Fe/Al/Ag and Al-Ni dissimilar metals J. Optoelectron. Adv. Mater. 13 1176
Hagimoto Y, Ugajin H, Miyakoshi D, Iwamoto H, Muraki Y and Orii T 2008 Evaluation of the plasmaless gaseous etching process Solid State Phenom. 134 7
Kompa K L and Walther H 2013 High-Power Lasers and Applications: Proceedings of the Fourth Colloquium on Electronic Transition Lasers in Munich, June 20–22, 1977 (Springer: Berlin)
Oh H J, Lee J H, Lee M S, Shin W G, Kang S Y, Kim G D and Ko D H 2014 NF3/NH3 dry cleaning mechanism inspired by chemical and physical surface modification of Si, SiO2, and Si3N4 ECS Trans. 61 1
Reichardt H, Frenzel A and Schober K 2001 Environmentally friendly wafer production: NF 3 remote microwave plasma for chamber cleaning Microelectron. Eng. 56 73
Tsai W T 2008 Environmental and health risk analysis of nitrogen trifluoride (NF3), a toxic and potent greenhouse gas J. Hazard. Mater. 159 257
Robson J I, Gohar L K, Hurley M D, Shine K P and Wallington T J 2006 Revised IR spectrum, radiative efficiency and global warming potential of nitrogen trifluoride Geophys. Res. Lett. 33
Blanco F, Alkorta I, Rozas I, Solimannejad M and Elguero J 2011 A theoretical study of the interactions of NF 3 with neutral ambidentate electron donor and acceptor molecules Phys. Chem. Chem. Phys. 13 674
Seif A, Bagherzadeh R, Goodarzi M and Azizi K 2013 Ab initio study of 1: 1 complexes of nitrogen trifluoride with nitrous oxide and carbon dioxide in vacuo J. Chem. Sci. 125 1277
McNamara B, Scheele R, Kozelisky A and Edwards M 2009 Thermal reactions of uranium metal, UO 2, U 3 O 8, UF 4, and UO 2 F 2 with NF 3 to produce UF 6 J. Nucl. Mater. 394 166
Jacob D 1999 Introduction to Atmospheric Chemistry (Princeton University Press: Princeton)
Finlayson-Pitts B J and Pitts J N 2000 Chemistry of the Lower and Upper Atmosphere (Academic Press: California)
Lu Q B 2013 Cosmic-ray-driven reaction and greenhouse effect of halogenated molecules: culprits for atmospheric ozone depletion and global climate change Int. J. Mod. Phys. B 27 1350073
Molina M J and Rowland F S 1974 Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone Nature 249 810
Torkpoor I, Heidari M, Janjanpour Nezhad, Salehi N, Gharibzadeh F and Edjlali L 2018 Insight into Y@X2B8 (Y = Li, CO2 and Li-CO2, X = Be, B and C) nanostructures: a computational study Chem. Rev. Lett. 1 2
Shiryaeva E S, Tyurin D A and Feldman V I 2016 Mechanisms of radiation-induced degradation of CFCl3 and CF2Cl2 in noble-gas matrixes: an evidence for “Hot” ionic channels in the solid phase J. Phys. Chem. A 120 7847
Ingólfsson O, Weik F and Illenberger E The reactivity of slow electrons with molecules at different degrees of aggregation: gas phase, clusters and condensed phase Int. J. Mass Spectrom. Ion Process. 155 1
Kiendler A, Matejcik S, Skalny J D, Stamatovic A and Märk T D 1996 Dissociative electron attachment to using a high-resolution crossed-beams technique J. Phys. B 29 6217
Solovev S, Kusmierek D O, Madey and T E 2004 Negative ion formation in electron-stimulated desorption of CF2Cl2 coadsorbed with polar NH3 on Ru (0001) J. Chem. Phys. 120 968
Poterya V, Kočišek J, Lengyel J, Svrčková P, Pysanenko A, Hollas D, Slavíček P and Fárník M 2014 Clustering and photochemistry of freon CF2Cl2 on argon and ice nanoparticles J. Phys. Chem. A 118 4740
Seif A, Ebrahimi S, Vessally E and Goodarzi M 2013 Comparative study on the stabilities and properties of heterodimers containing the intermolecular interactions of CF2Cl2 with the isoelectronic and isostructure species of N2O and CO2 Struct. Chem. 24 1737
de Paul Nziko V and Scheiner S 2015 Intramolecular S··· O chalcogen bond as stabilizing factor in geometry of substituted phenyl-SF3 molecules, (n.d.) J. Org. Chem. 80 2356
Ollila A 2013 Analyses of IPCC’s warming calculation results J. Chem. Biol. Phys. Sci. 3 2912
Møller C and Plesset M S 1934 Note on an approximation treatment for many-electron systems Phys. Rev. 46 618
Dunning Jr T H, Peterson K A and Wilson A K 2001 Gaussian basis sets for use in correlated molecular calculations. X. The atoms aluminum through argon revisited J. Chem. Phys. 114 9244
Nziko V de P N and Scheiner S 2014 Chalcogen bonding between tetravalent SF4 and amines J. Phys. Chem. A 118 10849
Becke A D 1993 Becke’s three parameter hybrid method using the LYP correlation functional J. Chem. Phys. 98 5648
Zhao Y, Schultz N E and Truhlar D G 2006 Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions J. Chem. Theory Comput. 2 364
Cohen A J, Mori-Sánchez P and Yang W 2011 Challenges for density functional theory Chem. Rev. 112 289
Boys S F and de Bernardi F 1970 The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors Mol. Phys. 19 553
Bader R F W 1990 Atoms in Molecules. A Quantum Theory (Clarendon: Oxford)
Bader R F W, Carroll M T, Cheeseman J R, Chang C 1987 Properties of atoms in molecules: atomic volumes, J. Am. Chem. Soc. 109 7968
Biegler-Konig F, Schonbohm J and Bayles D 2001 AIM2000—a program to analyze and visualize atoms in molecules J. Comput. Chem. 22 545
Glendening E D 1996 Natural energy decomposition analysis: explicit evaluation of electrostatic and polarization effects with application to aqueous clusters of alkali metal cations and neutrals J. Am. Chem. Soc. 118 2473
Reed A E, Curtiss L A and Weinhold F 1988 Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint Chem. Rev. 88 899
Schmidt M W, Baldridge K K, Boatz J A, Elbert S T, Gordon M S, Jensen J H, Koseki S, Matsunaga N, Nguyen K A, Su S J 1993, EXECUTION OF GAMESS BEGUN 21: 01: 54 LT 10-JUL-2006 J. Comput. Chem. 14 1347
Politzer P, Murray J S and Clark T 2013 Halogen bonding and other σ-hole interactions: a perspective Phys. Chem. Chem. Phys. 15 11178
Koch U and Popelier P L A 1995 Characterization of CHO hydrogen bonds on the basis of the charge density J. Phys. Chem. 99 9747
Wolters L P and Bickelhaupt F M 2012 Halogen bonding versus hydrogen bonding: a molecular orbital perspective ChemistryOpen 1 96
Murray J S, Paulsen K and Politzer P 1994 Molecular surface electrostatic potentials in the analysis of non-hydrogen-bonding noncovalent interactions J. Chem. Sci. 106 267
Metrangolo P, Neukirch H, Pilati T and Resnati G 2005 Halogen bonding based recognition processes: a world parallel to hydrogen bonding Acc. Chem. Res. 38 386
Wei Y, Li Q, Li W, Cheng J and McDowell S A C 2016 Influence of the protonation of pyridine nitrogen on pnicogen bonding: competition and cooperativity Phys. Chem. Chem. Phys. 18 11348
Reed A E, Curtiss L A and Weinhold F 1988 Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint Chem. Rev. 88 899
Banerjee P and Chakraborty T 2018 Weak hydrogen bonds: insights from vibrational spectroscopic studies Int. Rev. Phys. Chem. 37 83
Seif A and Massahi S 2014 Theoretical study on the properties and stabilities of complexes formed between SO 4 (C2v) and isostructure species of CO2, CS2, and SCO J. Mol. Model. 20 2488
Parr RG and Yang W 1989 Density-Functional Theory of Atoms and Molecules (Oxford Univ. Press: New York)
Hudgens J W, Johnson III R D, Tsai B P and Kafafi S A 1990 Experimental and ab initio studies of electronic structures of the trichloromethyl radical and cation J. Am. Chem. Soc. 112 5763
Pourreza A, Askari S, Rashidi A, Seif A and Kooti M 2019 Highly efficient SO3Ag-functionalized MIL-101 (Cr) for adsorptive desulfurization of the gas stream: experimental and DFT study Chem. Eng. J. 363 73
Tehrani N H M H, Alivand M S, Maklavany D M, Rashidi A, Samipoorgiri M, Seif A and Yousefian Z 2019 Novel asphaltene-derived nanoporous carbon with NS-rich micro-mesoporous structure for superior gas adsorption: experimental and DFT study Chem. Eng. J. 358 1126
Hensen K, Stumpf T, Bolte M, Näther C and Fleischer H 1998 Experimental investigations and ab initio studies on hexacoordinated complexes of dichlorosilane J. Am. Chem. Soc. 120 10402
Gharakhani F, Vessally E, Esrafili M D and Seif A 2015 The interaction energies between glycoluril clip and thiophenol derivatives using density functional theory calculations J. Sulfur Chem. 36 351
Acknowledgements
We are grateful to the University of Kurdistan Research Council for partial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdi, N., Seif, A., Azizi, K. et al. Insight into 1:1 complexes of H2O with NF3 and CF2Cl2: a quantum chemical approach. J Chem Sci 132, 52 (2020). https://doi.org/10.1007/s12039-020-1753-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-020-1753-z