- 1College of Animal Science and Technology, Henan University of Science and Technology, Luoyang, China
- 2Key Laboratory of Molecular Pathogen and Immunology of Animal of Luoyang, Luoyang, China
- 3Groupe de Recherche en Écologie Buccale, Faculté de Médecine Dentaire, Université Laval, Quebec, QC, Canada
- 4College of Life Science, Luoyang Normal University, Luoyang, China
Streptococcus suis (S. suis) and Actinobacillus pleuropneumoniae (A. pleuropneumoniae) are primary swine pathogens that have been frequently co-isolated from pigs suffering from severe respiratory disease. The purpose of this study was to investigate the biological impacts of the interactions between S. suis and A. pleuropneumoniae. A single- and dual-species culture model was established in vitro via S. suis HA9801 (serotype 2) and A. pleuropneumoniae CVCC265 (serotype 1). The single or mixed biofilms were imaged by confocal laser scanning microscopy. The biomass and viable cells in biofilms were quantified by crystal violet staining and determination of colony-forming units. The antibiotic susceptibility was determined by a microdilution broth method. The differences in gene transcription in pure- or mixed-species biofilms of S. suis and A. pleuropneumoniae was evaluated by quantitative PCR. S. suis and A. pleuropneumoniae formed two-species biofilms when co-cultured in vitro. When co-cultured with S. suis, biofilm formation by A. pleuropneumoniae was significantly increased with the absence of NAD that is necessary for the growth of A. pleuropneumoniae. Moreover, compared with monocultures, the antibiotic resistance of S. suis and A. pleuropneumoniae was both enhanced in the co-culture model. When grown in dual-species biofilms, for A. pleuropneumoniae, genes associated with virulence factors, including exotoxins and adhesins, were significantly upregulated. For S. suis, virulence factor-related genes cps2, gdh, mrp, and sly were highly induced. These results suggest that the interspecies interactions between S. suis and A. pleuropneumoniae may be cooperative under specific conditions and may play an important role in the disease progression and persistent infection.
Introduction
Respiratory diseases constitute the most important health issues affecting the swine industry worldwide and are often referred to as the porcine respiratory disease complex (PRDC) (Qiao et al., 2011). In general, PRDC causes lung lesions, which, in turn, results in substantial economic losses and impaired animal welfare (Fablet et al., 2012). PRDC is multifactorial in nature and is triggered by mixed infections involving primary and secondary pathogens (Cheong et al., 2017). Clinically, Streptococcus suis (S. suis) and Actinobacillus pleuropneumoniae (A. pleuropneumoniae) are two important respiratory pathogens frequently detected in the PRDC (Opriessnig et al., 2011). In recent years, there have been many cases of mixed infections associated with the presence of S. suis and A. pleuropneumoniae in China. It is more than likely that the different bacteria or viruses coexisting in PRDC interact and exacerbate the pathogenesis of the disease. However, few research groups investigated the interactions between S. suis and A. pleuropneumoniae, except for a study reporting that these two bacterial species form polymicrobial biofilms in vitro (Ramirez-Castillo et al., 2018).
The formation of biofilm is considered as a survival strategy for bacterial pathogens. Bacteria in biofilms are embedded in an extracellular matrix, which reduces their susceptibility to antibiotic and host immune system (Vuotto and Donelli, 2019). This is a major reason why biofilm-related infections are difficult to eradicate. During a chronic infection, bacteria are able to switch between planktonic and biofilm lifestyle (Kathju et al., 2009; Lister and Horswill, 2014). The key role of biofilms have been confirmed in many diseases such as endocarditis, periodontitis, and otitis media (Kobayashi et al., 2005). Multi-species biofilms may be the most significant lifestyle of microbes in vivo and bacteria in such polymicrobial biofilms are more difficult to eradicate (Wang et al., 2018). In multi-species biofilms, microorganisms will enhance and ensure their survival and reproduction through communication, competition, or cooperation (Yang et al., 2011). In the present study, we hypothesized that there are synergistic interactions between S. suis and A. pleuropneumoniae in dual-species biofilms, resulting in enhanced biofilm formation ability, enhanced antibiotic resistance, and upregulated virulence factor gene expression.
Materials and Methods
Bacterial Strains and Growth Conditions
S. suis HA9801 (SS) was isolated from a diseased pig in Jiangsu Province, China, and identified to be S. suis serotype 2. All experiments in this study were approved by the Experimental Animal Monitoring Committee of Henan University of Science and Technology and carried out accordingly. A. pleuropneumoniae CVCC 265 (APP) was purchased from China Veterinary Culture Collection Center (CVCC). S. suis was grown in tryptic soy broth (TSB) or plated on TSA. A. pleuropneumoniae was cultivated in the same media supplemented with 0.1 μg/mL of β-nicotinamide-adenine-dinucleotide (NAD). Bacterial cultures were incubated at 37°C. All experiments described below were replicated in biological triplicate.
Planktonic Growth Assays of Mono- and co-Cultures
Bacterial colonies of S. suis and A. pleuropneumoniae were inoculated separately in solid media and grown overnight to mid-exponential phase in TSB + 0.1% NAD medium. The bacterial cultures were diluted in fresh medium to obtain an optical density at 660 (OD660) of 0.03. Mono-culture of S. suis or A. pleuropneumoniae and co-culture of mixture (1:1 ratio) were inoculated in TSB + 0.1% NAD medium for 24 h. Samples were taken at 0, 2, 4, 6, 8, 10, 12, and 24 h, serially diluted in sterile PBS, and then plated on TSA or TSA + 0.1% NAD medium to discriminate S. suis or A. pleuropneumoniae, respectively, and the viable cell counts were enumerated.
For the mixed culture, the Competitive Index (CI) was calculated according to the formula: (A. pleuropneumoniae/S. suis)output/(A. pleuropneumoniae/S. suis)input. The output and input samples were assessed by plating onto selective medium at different time points, respectively. The Relative Increase Ratio (RIR) was similar to CI, and was calculated from the corresponding growth results obtained from single cultures of each strain (Macho et al., 2007). A positive CI value suggests a competitive advantage for A. pleuropneumoniae and vice versa. Only CIs that are statistically different from the RIRs at the same growth stages can be recognized as the result of prominent competition between species (Macho et al., 2007).
Biofilm Formation by Single and Mixed Cultures
Biofilm production was quantified by crystal violet staining as described previously (Bragonzi et al., 2012). Briefly, overnight cultures of S. suis and A. pleuropneumoniae were diluted 1/100 in fresh TSB broth supplemented with NAD, and inoculated individually or at several different ratios in 96-well plates. After 24 h incubation, the medium and planktonic bacteria were removed, and each well was gently washed twice with sterile PBS. Methanol was used to fix the attached bacteria for 15 min. The plates were then air-dried and the biofilms were stained with crystal violet (0.1%). After 20 min, the excess dye was discarded and plates were washed twice with sterile PBS prior to adding 200 μl of 95% ethanol to the wells to dissolve the biofilms. The optical density at 620 nm (OD620) was measured using a microplate reader.
Biofilms were prepared in the 96-well plates as described above. Biofilms were washed twice with sterile PBS, and then bacteria were detached and homogenized in 100 μl of sterile PBS by weak sonication for 4 min. TSA or TSA + 0.1% NAD medium was used to discriminate S. suis or A. pleuropneumoniae, respectively, and then viable cells were enumerated (Chan et al., 2017).
Confocal Laser Scanning Microscopy
Chamber slides were inoculated with bacterial suspension (S. suis alone, A. pleuropneumoniae alone and combination in 1:1 ratio) for 24 h at 37°C. The slides were washed twice with PBS to remove the medium and unattached bacteria. Samples was stained with SYTO 9 solution following the manufacturer’s instructions from LIVE/DEADTM BacLightTM Bacterial Viability Kit (Thermo Fischer Scientific, Inc., Waltham, MA, United States), and then washed with PBS (Tawakoli et al., 2013). Biofilms were observed using a Zeiss LSM800 CLSM (Carl Zeiss, Jena, Germany).
Antibiotic Susceptibility Testing
The in vitro antibiotic susceptibility of S. suis and A. pleuropneumoniae was determined by a twofold dilution method in microplates following the guidelines of Clinical and Laboratory Standards Institute. Antibiotic serial dilutions were made up in culture medium, and 100 μl was transferred to the wells. Overnight cultures of S. suis and A. pleuropneumoniae were diluted at 1:100 with TSB contain in 0.1% NAD, and 100 μl of S. suis, A. pleuropneumoniae, or combination in 1:1 were added to 96-well plates and incubated at 37°C for 24 h. The minimum inhibitory concentration (MIC) values were determined by reading the optical density and visual observation of the turbidity.
The minimum biofilm eradication concentration (MBEC) values were also determined. Biofilms were prepared in the 96-well plates as described above. Wells were washed twice with PBS and antibiotic serial dilutions made up in TSB containing 0.1% NAD were transferred (200 μl) to the wells. The plates were incubated for another 24 h. The MBEC values were determined by plating samples on culture medium plates.
Virulence Factor Gene Expression in Mixed Biofilm
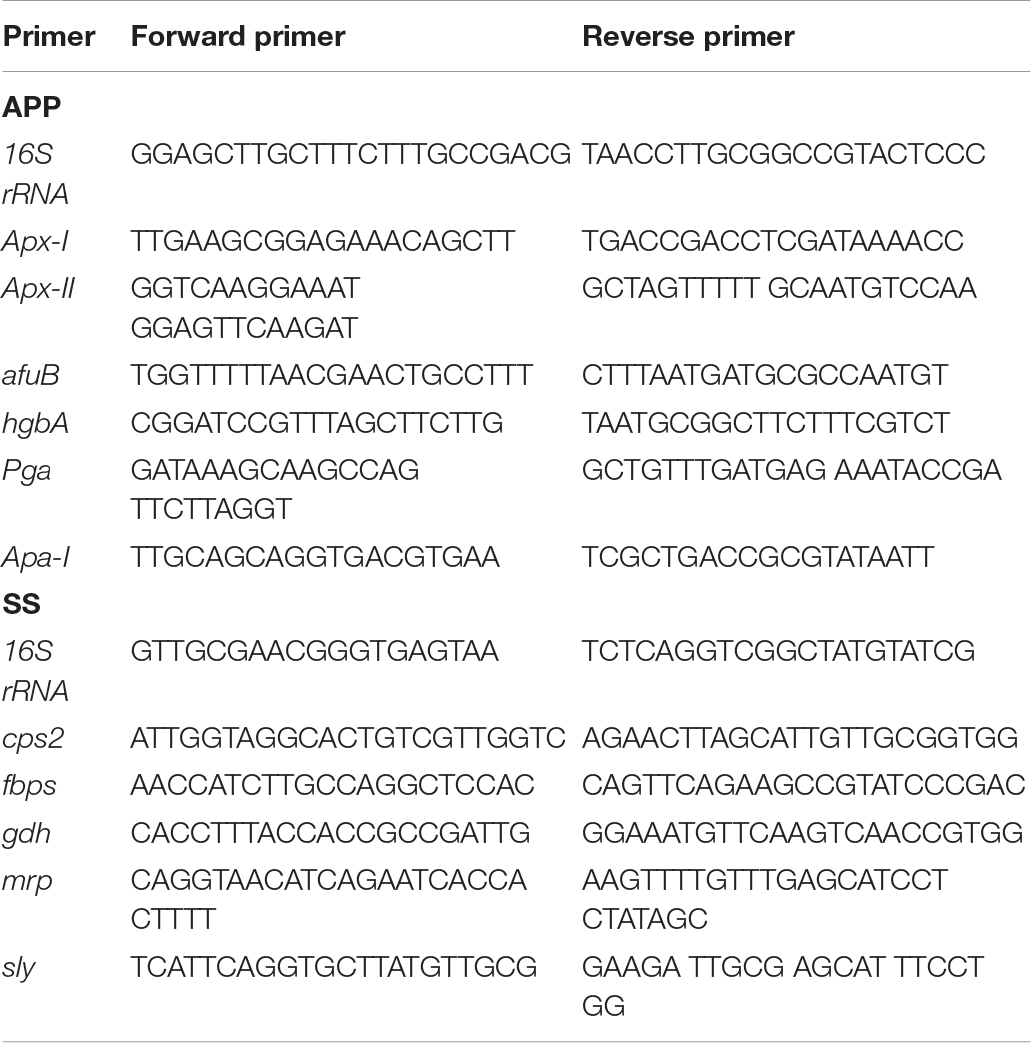
Real-time PCR was used to assess the relative expression of virulence factor genes in A. pleuropneumoniae or S. suis when grown alone or in mixed biofilms. Biofilms were prepared in 24-well plates as described above, washed twice with PBS, detached by sonicating, and then collected. RNA was extracted by the Trizol method (Pompilio et al., 2015). gDNA removal and cDNA synthesis were performed using PrimeScriptTM RT reagent Kit with gDNA Eraser (TaKaRa) according to the manufacturer’s protocol. Real-time PCR assay was performed with TB Green® Premix Ex TaqTM (TaKaRa) following the manufacturer’s instructions. The primers used are listed in Table 1. The 16S rRNA was used as the house-keeping gene for normalization. Relative expression levels were determined by the (ΔΔCt) method.
Statistical Analysis
GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, United States) was used for data analysis. Results were obtained from three independent experiments and all values were expressed as means ± standard deviation. Differences between mean values were evaluated by Student’s t-test. P-value of 0.05 or less was considered statistically significant.
Results
Competition Between S. suis and A. pleuropneumoniae in Planktonic co-Cultures
To study the interactions between S. suis and A. pleuropneumoniae in planktonic co-cultures, the growth curves of single and mixed cultures were compared, and the results are shown in Figure 1A. The kinetics analysis showed that the growth of S. suis in mixed culture was negatively affected from 8 to 24 h, while A. pleuropneumoniae growth was not clearly affected when co-cultured with S. suis. To further estimate the differences in growth kinetics between S. suis and A. pleuropneumoniae in single or mixed cultures, CI and RIR indexes were calculated. As shown in Figure 1B, a positive CI index of A. pleuropneumoniae versus S. suis was always observed, indicating a competitive advantage for A. pleuropneumoniae over S. suis in co-cultures. The CI was significantly higher than the RIR between 8 and 24 h (p < 0.05), suggesting a noticeable negative effect of A. pleuropneumoniae on S. suis growth.
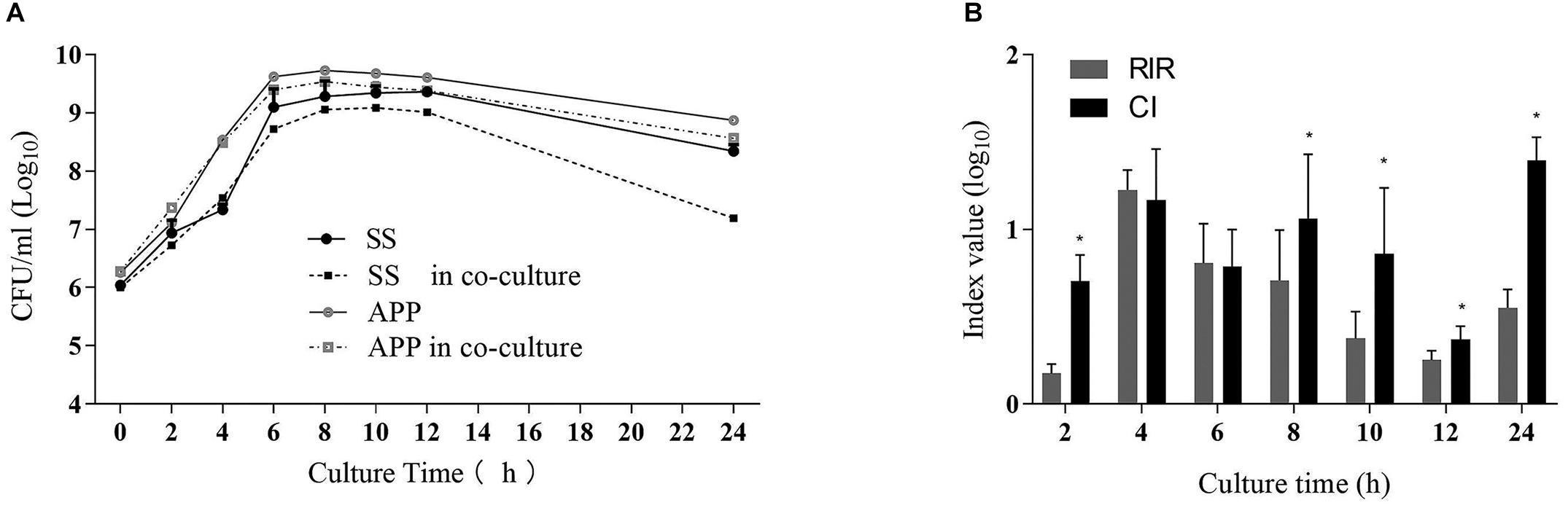
Figure 1. Single- and dual-species planktonic growth kinetics and competition index values. S. suis and A. pleuropneumoniae were inoculated in TSB + 0.1% NAD medium for 24 h in mono and mixed cultures. Growth curves were monitored by colony-forming unit counts (CFU) after plating on TSA or TSA + 0.1% NAD medium to discriminate S. suis or A. pleuropneumoniae respectively. (A) Growth curves of S. suis in single culture (SS) and in co-culture with A. pleuropneumoniae (SS in co-culture). Growth curves of A. pleuropneumoniae in pure culture (APP) and in co-culture with S. suis (APP in co-culture). (B) Competitive index (CI) and Relative Increase Ratio (RIR) of S. suis and A. pleuropneumoniae obtained from single culture and co-culture were calculated as described in section Materials and Methods. Error bars represent standard deviation of three separate assays. * p < 0.05.
Formation of Mixed Biofilm of S. suis and A. pleuropneumoniae
Biofilm formation by S. suis and A. pleuropneumoniae in single and dual culture in 96-well plate was assessed by crystal violet staining and viable count, and the results are shown in Figure 2. Both S. suis and A. pleuropneumoniae formed important biofilms when grown in TSB supplemented with NAD (Figure 2A). However, in the absence of NAD, A. pleuropneumoniae couldn’t form biofilms (Figure 2B). Under favorable growth conditions(supplement with NAD)for A. pleuropneumoniae, mixed biofilms with S. suis were formed (Figure 2C). Moreover, based on results of crystal violet staining and determination of CFU, A. pleuropneumoniae was able to grow and form a dual-species biofilm without the addition of NAD when grown in the presence of S. suis (Figure 2D).
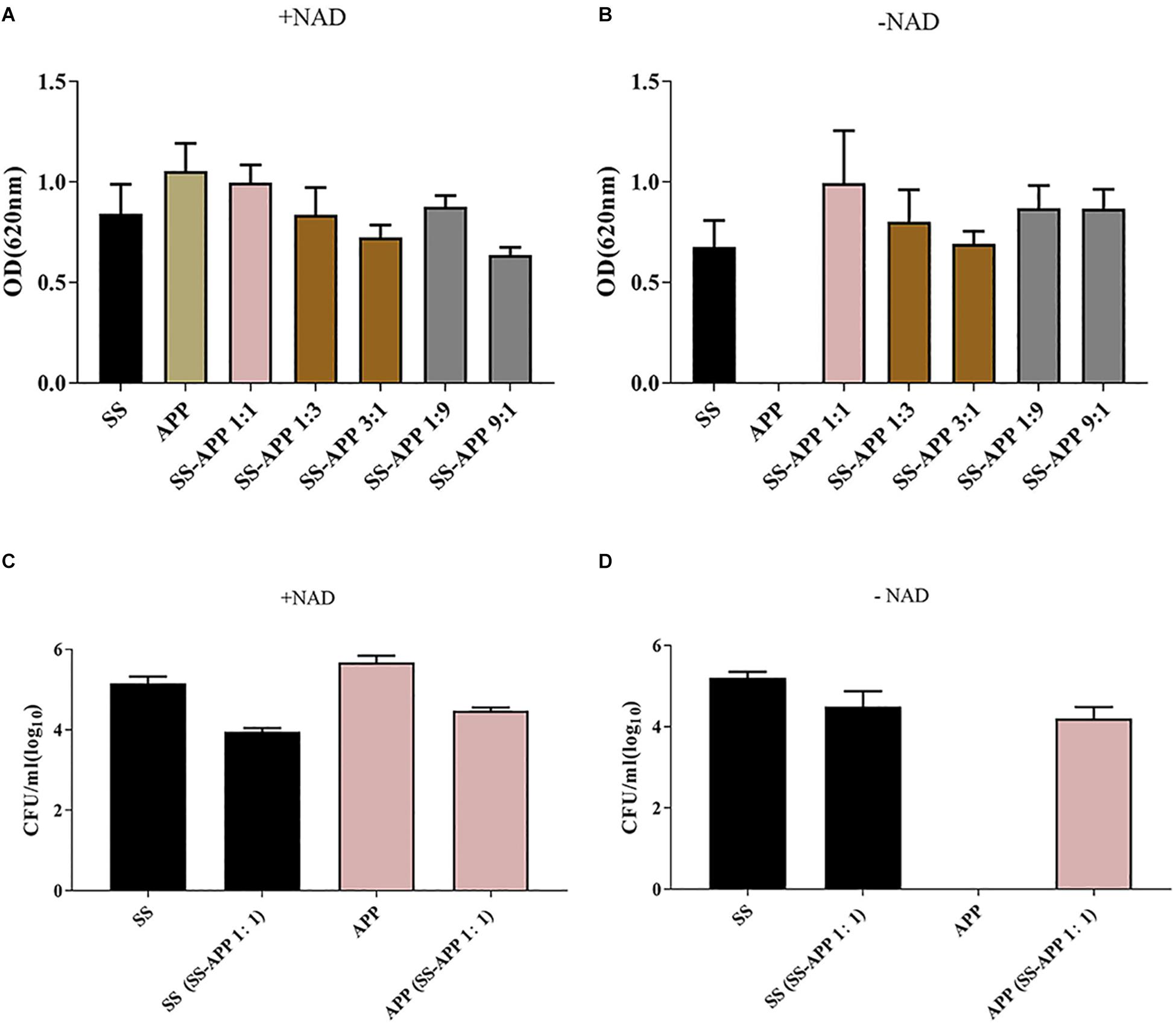
Figure 2. Biofilm formation by S. suis and A. pleuropneumoniae in mono and mixed cultures. Single or mixed biofilms formed by S. suis and A. pleuropneumoniae in TSB media (A) or in TSB + 0.1% NAD medium (B) were quantified by crystal violet staining. The colony-forming units (CFU) for S. suis and A. pleuropneumoniae in single or mixed biofilms were determined in TSB media (C) or in TSB + 0.1% NAD medium (D).
By using confocal laser scanning microscopy (Figure 3), it could be confirmed that both S. suis and A. pleuropneumoniae were able to form robust single- and dual-species biofilms in vitro.
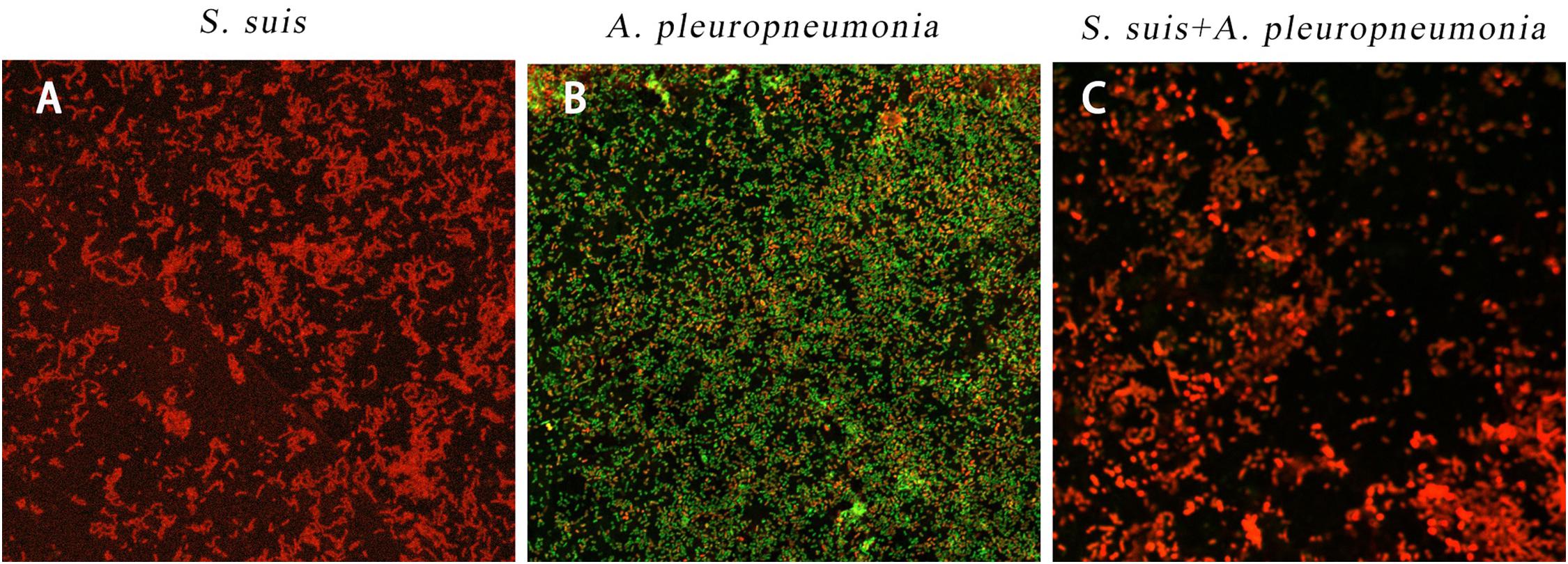
Figure 3. Biofilm formation of single and mixed species imaged by confocal laser scanning microscopy at × 63 magnification after 24 h inoculation. S. suis alone (A), A. pleuropneumoniae alone (B), or S. suis and A. pleuropneumoniae together (C).
Antibiotic Susceptibility
The in vitro antibiotic susceptibility of S. suis and A. pleuropneumoniae, individually and in combination to several antimicrobial drugs, was determined (Table 2). For tylosin tartrate, when in co-culture with the other, the MBEC of S. suis increased from 1.25 to 80 μg/ml, and the MBEC of A. pleuropneumoniae increased from 40 to 80 μg/ml. For tilmicosin, when in co-culture, the MIC and MBEC of S. suis or A. pleuropneumoniae were twice as high as that in pure culture. In general, S. suis and A. pleuropneumoniae in mixed biofilms showed increased resistance to tylosin tartrate and tilmicosin with higher values of MBEC in comparison to single-species biofilms.
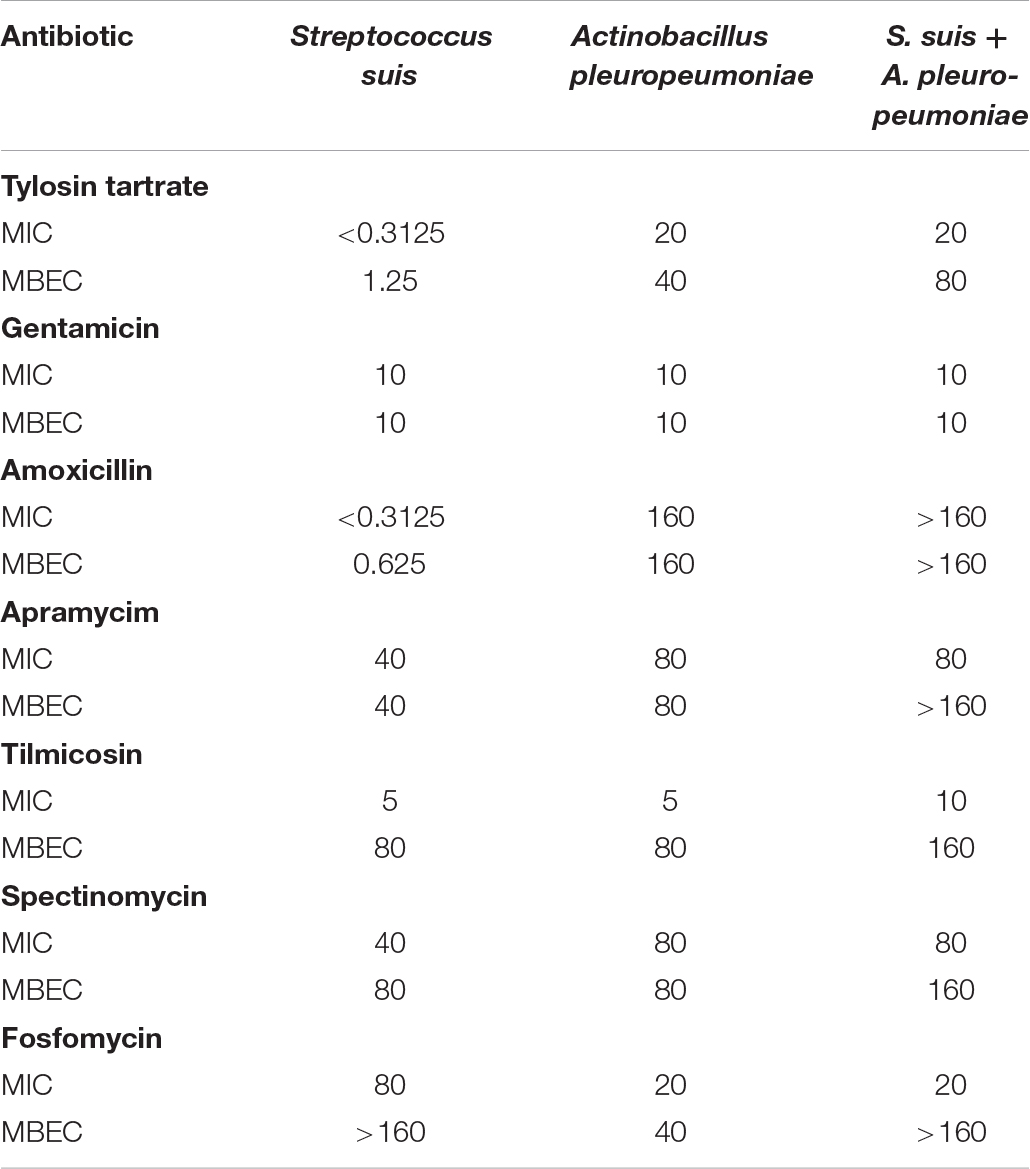
Table 2. In vitro susceptibility of pure culture and co-culture of S. suis and A. pleuropneumoniae in planktonic growth and biofilm to seven clinically relevant antibiotics.
Differential Gene Expression in Mono- and Dual-Species Biofilms
As shown in Figure 4, in comparison with the single species biofilms, the virulence factor genes in the two-species biofilms were overall upregulated. For A. pleuropneumoniae, compared with mono-species biofilms, ApxI and ApxII, codifying for exotoxin, were upregulated by 10.65- and 22.58-fold, respectively, afuB involved in biofilm formation was upregulated by 8.69-fold, apaI associated with adhesin was upregulated by 5.73-fold, and hgbA involved in iron uptake was upregulated by 18.3-fold (Figure 4A). For S. suis, virulence factor related genes cps2, gdh, mrp, and sly were significantly upregulated by 2. 61-, 2. 23-, 3. 81-, and 2.26-fold, respectively, with no statistical differences of fbps gene (Figure 4B). The results may indicate that co-culture may modulate the bacterial virulence in mixed biofilm.
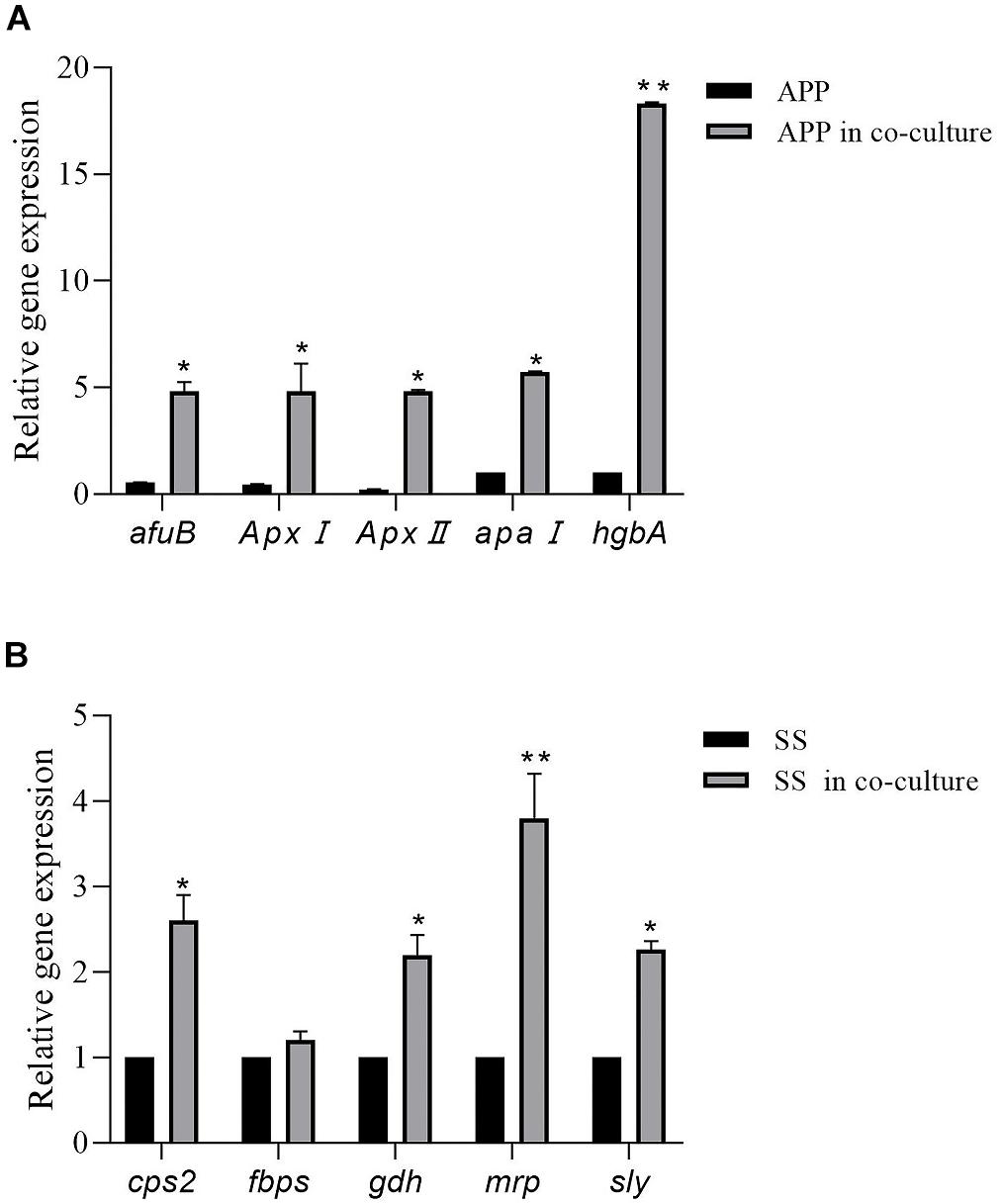
Figure 4. Relative expression levels of A. pleuropneumoniae virulence factor genes (ApxI, ApxII, afuB, apaI, and hgbA) in mixed biofilm (A). Relative expression levels of S. suis virulence factor genes (cps2, fbps, gdh, mrp, and sly) in mixed biofilm (B). Error bars represent standard deviation of three separate assays. * p < 0.05; ** p < 0.01.
Discussion
Polymicrobial respiratory diseases remain a major threat in the swine industry worldwide. Pathogens associated with PRDC include swine influenza virus (SIV), porcine circovirus type 2 (PCV2), porcine reproductive and respiratory syndrome virus (PRRSV), S. suis, A. pleuropneumoniae, Mycoplasma hyopneumoniae, and Haemophilus parasuis. Bacterial–viral co-infections were reported to exacerbate the pathogenicity (Opriessnig et al., 2011). For instance, co-infections of M. hyopneumoniae and SIV lead to the exacerbation of the clinical signs (Thacker et al., 1999), and during the co-infection of PRRSV and S. suis, the virulence of PRRSV was enhanced by S. suis and PRRSV increased the susceptibility of pigs to S. suis infection (Thanawongnuwech et al., 2000). S. suis and A. pleuropneumoniae are two important pathogens associated with PRDC, and their co-occurrence in the same site of infection has been frequently reported (Opriessnig et al., 2011), although the interactions between them and the host during coinfection have not been previously investigated. The present study examined the interactions between S. suis and A. pleuropneumoniae in planktonic and biofilm cultures, and specifically explored whether these interactions provide greater fitness than single cultures, which is helpful to better understand the possible role of coinfection in the pathogenesis.
Studies on microbial interactions under planktonic growth conditions have shown that species can coexist with other microbes competing in the same repository through multiple mechanisms (Hibbing et al., 2010). It was reported that the consumption of limited nutriments may impact the interactions process between species in co-culture models (Sibley et al., 2008). We demonstrated that S. suis has no effect on the growth of A. pleuropneumoniae in planktonic culture, while a significant negative impact of A. pleuropneumoniae on S. suis growth was found during the stationary phase of bacterial growth.
When grown in mixed cultures, the exoproducts of A. pleuropneumoniae may be the disadvantageous cause of S. suis in planktonic bacteria. The CI and RIR indicated that A. pleuropneumoniae surpasses S. suis in exponential and stationary phases of bacterial growth. The competition for limited nutrients and space may lead to the antagonistic effects among microorganisms within a community (Harrison, 2007).
Crystal violet staining and colony-forming unit results showed that both S. suis and A. pleuropneumoniae formed strong biofilms when grown in single or mixed culture, and the CSLM images provided additional evidence. Interestingly, A. pleuropneumoniae was able to grow in the absence of NAD when co-cultured with S. suis. Our results confirm that the presence of S. suis promoted A. pleuropneumoniae biofilm growth under a hostile condition for A. pleuropneumoniae (without NAD supplementation). A variety of animal or human pathogens, such as Streptococcus mutans, Pseudomonas aeruginosa, Legionella pneumophila, Stenotrophomonas maltophilia, and Escherichia coli, have been reported that to form mixed biofilms, which help to improve their resistance, persistence, and pathogenicity (Yang et al., 2011). Recent studies on multi-species community behavior have shown that the presence of other bacterial species can modulate the virulence and the gene expression of pathogens. In the respiratory tract, the NAD supply, which is essential for A. pleuropneumoniae growth, is rather limited. However, A. pleuropneumoniae has overcome this deficiency in various ways, such as cell lysis, which releases nutrients into the surrounding environment (Chiers et al., 2010). In addition, polymicrobial biofilm formation with S. suis enables A. pleuropneumoniae to acquire this compound by cross-feeding.
We showed that a mixed biofilm of S. suis and A. pleuropneumoniae has an increased resistance to several antibiotics. In fact, it is well known that bacteria residing in biofilms have an increased resistance to antibiotics (Assavacheep and Rycroft, 2013), and multi-species biofilm is believed to provide an enhanced protection against antibiotic and host immune system (Armbruster et al., 2010). Some well-known mechanisms for enhancing multi-microbial biofilm resistance include upregulation and transfer of drug resistance genes, increased responsiveness through quorum sensing, and increased mutation level in antibiotic target molecules (Hoiby et al., 2010). Recent research of multi-species community behavior have reported that the virulence, biofilm formation, and the gene expression level of pathogens can be regulated by the presence of other species (Duan et al., 2003). To investigate this, quantitative PCR was performed to analyze differential gene expression in mixed biofilms. The RT-PCR results showed that genes of A. pleuropneumoniae, coding for exotoxin, biofilm formation, or iron uptake, respectively, were highly induced. Further, genes of cps2, gdh, mrp, and sly associated with virulence factors of S. suis were significantly upregulated. The result suggests that mixed biofilms may reinforce bacteria pathogenicity.
In conclusion, we found that co-culture may result in increased antibiotic resistance and upregulated virulence gene expression for S. suis and A. pleuropneumoniae in biofilms. It is likely that the interspecies interactions between S. suis and A. pleuropneumoniae are synergetic under specific conditions. Therefore, the interactions between the species in the biofilm community potentially influence the clinical course of disease. Our findings provide some relevant information that may affect the choice of antibiotics, and shed light on a new perspective for the treatment of mixed infection.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
YW and LY conceived and designed the experiments. SG, XD, and JL performed the experiments. YW, XD, and DG analyzed the data. LY and JL contributed reagents, materials, and analysis tools. YW and SG wrote the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFD0500100), the National Natural Science Foundation of China (31902309 and 31772761), Luoyang Normal University National project cultivation program, and Young Teacher Foundation of Luoyang Normal University.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Armbruster, C. E., Hong, W., Pang, B., Weimer, K. E., Juneau, R. A., Turner, J., et al. (2010). Indirect pathogenicity of Haemophilus influenzae and Moraxella catarrhalis in polymicrobial otitis media occurs via interspecies quorum signaling. mBio 1:e00102-10. doi: 10.1128/mBio.00102-10
Assavacheep, P., and Rycroft, A. N. (2013). Survival of Actinobacillus pleuropneumoniae outside the pig. Res. Vet. Sci. 94, 22–26. doi: 10.1016/j.rvsc.2012.07.024
Bragonzi, A., Farulla, I., Paroni, M., Twomey, K. B., Pirone, L., Lore, N. I., et al. (2012). Modelling co-infection of the cystic fibrosis lung by Pseudomonas aeruginosa and Burkholderia cenocepacia reveals influences on biofilm formation and host response. PLoS One 7:e52330. doi: 10.1371/journal.pone.0052330
Chan, C. L., Richter, K., Wormald, P. J., Psaltis, A. J., and Vreugde, S. (2017). Alloiococcus otitidis forms multispecies biofilm with Haemophilus influenzae: effects on antibiotic susceptibility and growth in adverse conditions. Front. Cell. Infect. Microbiol. 7:344. doi: 10.3389/fcimb.2017.00344
Cheong, Y., Oh, C., Lee, K., and Cho, K. H. (2017). Survey of porcine respiratory disease complex-associated pathogens among commercial pig farms in Korea via oral fluid method. J. Vet. Sci. 18, 283–289. doi: 10.4142/jvs.2017.18.3.283
Chiers, K., De Waele, T., Pasmans, F., Ducatelle, R., and Haesebrouck, F. (2010). Virulence factors of Actinobacillus pleuropneumoniae involved in colonization, persistence and induction of lesions in its porcine host. Vet. Res. 41:65. doi: 10.1051/vetres/2010037
Duan, K., Dammel, C., Stein, J., Rabin, H., and Surette, M. G. (2003). Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol. Microbiol. 50, 1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x
Fablet, C., Marois-Crehan, C., Simon, G., Grasland, B., Jestin, A., Kobisch, M., et al. (2012). Infectious agents associated with respiratory diseases in 125 farrow-to-finish pig herds: a cross-sectional study. Vet. Microbiol. 157, 152–163. doi: 10.1016/j.vetmic.2011.12.015
Harrison, F. (2007). Microbial ecology of the cystic fibrosis lung. Microbiology 153(Pt 4), 917–923. doi: 10.1099/mic.0.2006/004077-0
Hibbing, M. E., Fuqua, C., Parsek, M. R., and Peterson, S. B. (2010). Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25. doi: 10.1038/nrmicro2259
Hoiby, N., Bjarnsholt, T., Givskov, M., Molin, S., and Ciofu, O. (2010). Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 35, 322–332. doi: 10.1016/j.ijantimicag.2009.12.011
Kathju, S., Nistico, L., Hall-Stoodley, L., Post, J. C., Ehrlich, G. D., and Stoodley, P. (2009). Chronic surgical site infection due to suture-associated polymicrobial biofilm. Surg. Infect. 10, 457–461. doi: 10.1089/sur.2008.062
Kobayashi, Y., Sunobe, T., Kobayashi, T., Nagahama, Y., and Nakamura, M. (2005). Gonadal structure of the serial-sex changing gobiid fish Trimma okinawae. Dev. Growth Differ. 47, 7–13. doi: 10.1111/j.1440-169x.2004.00774.x
Lister, J. L., and Horswill, A. R. (2014). Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol. 4:178. doi: 10.3389/fcimb.2014.00178
Macho, A. P., Zumaquero, A., Ortiz-Martin, I., and Beuzon, C. R. (2007). Competitive index in mixed infections: a sensitive and accurate assay for the genetic analysis of Pseudomonas syringae-plant interactions. Mol. Plant Pathol. 8, 437–450. doi: 10.1111/j.1364-3703.2007.00404.x
Opriessnig, T., Gimenez-Lirola, L. G., and Halbur, P. G. (2011). Polymicrobial respiratory disease in pigs. Anim. Health Res. Rev. 12, 133–148. doi: 10.1017/S1466252311000120
Pompilio, A., Crocetta, V., De Nicola, S., Verginelli, F., Fiscarelli, E., and Di Bonaventura, G. (2015). Cooperative pathogenicity in cystic fibrosis: Stenotrophomonas maltophilia modulates Pseudomonas aeruginosa virulence in mixed biofilm. Front. Microbiol. 6:951. doi: 10.3389/fmicb.2015.00951
Qiao, S., Feng, L., Bao, D., Guo, J., Wan, B., Xiao, Z., et al. (2011). Porcine reproductive and respiratory syndrome virus and bacterial endotoxin act in synergy to amplify the inflammatory response of infected macrophages. Vet. Microbiol. 149, 213–220. doi: 10.1016/j.vetmic.2010.11.006
Ramirez-Castillo, F. Y., Loera-Muro, A., Vargas-Padilla, N. D., Moreno-Flores, A. C., Avelar-Gonzalez, F. J., Harel, J., et al. (2018). Incorporation of Actinobacillus pleuropneumoniae in preformed biofilms by Escherichia coli isolated from drinking water of swine farms. Front. Vet. Sci. 5:184. doi: 10.3389/fvets.2018.00184
Sibley, C. D., Duan, K., Fischer, C., Parkins, M. D., Storey, D. G., Rabin, H. R., et al. (2008). Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 4:e1000184. doi: 10.1371/journal.ppat.1000184
Tawakoli, P. N., Al-Ahmad, A., Hoth-Hannig, W., Hannig, M., and Hannig, C. (2013). Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin. Oral Investig. 17, 841–850. doi: 10.1007/s00784-012-0792-3
Thacker, E. L., Halbur, P. G., Ross, R. F., Thanawongnuwech, R., and Thacker, B. J. (1999). Mycoplasma hyopneumoniae potentiation of porcine reproductive and respiratory syndrome virus-induced pneumonia. J. Clin. Microbiol. 37, 620–627. doi: 10.1128/jcm.37.3.620-627.1999
Thanawongnuwech, R., Brown, G. B., Halbur, P. G., Roth, J. A., Royer, R. L., and Thacker, B. J. (2000). Pathogenesis of porcine reproductive and respiratory syndrome virus-induced increase in susceptibility to Streptococcus suis infection. Vet. Pathol. 37, 143–152.
Vuotto, C., and Donelli, G. (2019). Novel treatment strategies for biofilm-based infections. Drugs 79, 1635–1655. doi: 10.1007/s40265-019-01184-z
Wang, L., Li, Y., Wang, L., Zhang, H., Zhu, M., Zhang, P., et al. (2018). Extracellular polymeric substances affect the responses of multi-species biofilms in the presence of sulfamethizole. Environ. Pollut. 235, 283–292. doi: 10.1016/j.envpol.2017.12.060
Keywords: Streptococcus suis, Actinobacillus pleuropneumoniae, mixed biofilm, antibiotic susceptibility, swine
Citation: Wang Y, Gong S, Dong X, Li J, Grenier D and Yi L (2020) In vitro Mixed Biofilm of Streptococcus suis and Actinobacillus pleuropneumoniae Impacts Antibiotic Susceptibility and Modulates Virulence Factor Gene Expression. Front. Microbiol. 11:507. doi: 10.3389/fmicb.2020.00507
Received: 05 November 2019; Accepted: 09 March 2020;
Published: 07 April 2020.
Edited by:
Sujogya Kumar Panda, KU Leuven, BelgiumCopyright © 2020 Wang, Gong, Dong, Li, Grenier and Yi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Wang, wangyocean@163.com; Li Yi, lilili123168@163.com
 Yang Wang
Yang Wang Shenglong Gong
Shenglong Gong Xiao Dong
Xiao Dong Jinpeng Li
Jinpeng Li Daniel Grenier
Daniel Grenier Li Yi
Li Yi