Abstract
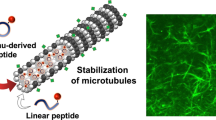
Microtubules, one of the major components of the cytoskeleton, play important roles as pathways for neuronal transport of cellular traffic. The loss of structural stability of microtubules causes detrimental effects on neurons and may contribute to several neurodegenerative diseases, such as Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease, etc. The triazolopyrimidine class compound cevipabulin is a synthetic microtubule-stabilizing agent that has recently emerged as a drug for the treatment of Alzheimer’s disease. However, the mechanism of microtubule stabilization by cevipabulin has not yet been revealed. Here, we explored the effect of cevipabulin on stabilizing microtubules polymerized from purified tubulins in vitro. We observed the effects of the concentration of microtubule-stabilizing drugs, incubation time, and modification of the cevipabulin structure on the stabilization of microtubules in comparison to those of the most commonly used anticancer drug, paclitaxel. This study will provide insight into the action of cevipabulin in the treatment of neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Howard J. Mechanics of motor proteins and the cytoskeleton. Sunderland, MA: Sinauer Associates Inc.; 2001.
Doodhi H, Prota AE, Rodriguez-Garcia R, Xiao H, Custar DW, Bargsten K, et al. Termination of protofilament elongation by eribulin induces lattice defects that promote microtubule catastrophes. Curr Biol. 2016;26:1713–21.
Scholey JM, Brust-Mascher I, Mogilner A. Cell division. Nature. 2003;422:746–52.
Ross JL, Ali MY, Warshaw DM. Cargo transport: molecular motors navigate a complex cytoskeleton. Curr Opin Cell Biol. 2008;20:41–7.
Alberts PB, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular biology of cell. New York: Garland Science; 2008.
Amos LA, Baker TS. The three-dimensional structure of tubulin protofilaments. Nature. 1979;279:607–12.
Mitchison T, Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312:237–42.
Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–54.
Itoh TJ, Hotani H. Microtubule-stabilizing activity of microtubule-associated proteins (MAPs) is due to increase in frequency of rescue in dynamic instability: shortening length decreases with binding of MAPs onto microtubules. Cell Struct Funct. 1994;19:279–90.
Dubey J, Ratnakaran N, Koushika SP. Neurodegeneration and microtubule dynamics: death by a thousand cuts. Front Cell Neurosci. 2015;9:343.
Giannakakou P, Nakano M, Nicolaou KC, O’Brate A, Yu J, Blagosklonny MV, et al. Enhanced microtubule-dependent trafficking and p53 nuclear accumulation by suppression of microtubule dynamics. Proc Natl Acad Sci USA. 2002;99:10855–60.
Brunden KR, Trojanowski JQ, Smith AB, Lee VMY, Ballatore C. Microtubule-stabilizing agents as potential therapeutics for neurodegenerative disease. Bioorg Med Chem. 2015;22:5040–9.
Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA. 1980;77:1561–5.
Dordunoo SK, Burt HM. Solubility and stability of taxol: effects of buffers and cyclodextrins. Int J Pharm. 1996;133:191–201.
Nicoletti MI, Colombo T, Rossi C, Monardo C, Stura S, Zucchetti M, et al. IDN5109, a taxane with oral bioavailability and potent antitumor activity. Cancer Res. 2000;60:842–6.
Sáez-Calvo G, Sharma A, Balaguer F, de A, Barasoain I, Rodríguez-Salarichs J, et al. Triazolopyrimidines are microtubule-stabilizing agents that bind the vinca inhibitor site of tubulin. Cell Chem Biol. 2017;24:737–50.
Beyer CF, Zhang N, Hernandez R, Vitale D, Lucas J, Nguyen T, et al. TTI-237: a novel microtubule-active compound with in vivo antitumor activity. Cancer Res. 2008;68:2292–300.
Zhang N, Ayral-Kaloustian S, Nguyen T, Afragola J, Hernandez R, Lucas J, et al. Synthesis and sar of [1,2,4]triazolo[1,5-a]pyrimidines, a class of anticancer agents with a unique mechanism of tubulin inhibition. J Med Chem. 2007;50:319–27.
Ballatore C, Brunden KR, Trojanowski JQ, Lee VMY, Smith AB. Non-naturally occurring small molecule microtubule-stabilizing agents: a potential tactic for CNS-directed therapies. ACS Chem Neurosci. 2017;8:5–7.
Beyer CF, Zhang N, Hernandez R, Vitale D, Nguyen T, Ayral-Kaloustian S, et al. The microtubule-active antitumor compound TTI-237 has both paclitaxel-like and vincristine-like properties. Cancer Chemother Pharmacol. 2009;64:681–9.
Nasrin SR, Kabir AMR, Konagaya A, Ishihara T, Sada K, Kakugo A. Stabilization of microtubules by cevipabulin. Biochem Biophys Res Commun. 2019;516:760–4.
Zhang N, Ayral-Kaloustian S, Nguyen T, Hernandez R, Lucas J, Discafani C, et al. Synthesis and SAR of 6-chloro-4-fluoroalkylamino-2-heteroaryl-5-(substituted)phenylpyrimidines as anti-cancer agents. Bioorg Med Chem. 2009;17:111–8.
Zhang N, Ayral-Kaloustian S, Nguyen T, Hernandez R, Beyer C. 2-Cyanoaminopyrimidines as a class of antitumor agents that promote tubulin polymerization. Bioorg Med Chem Lett. 2007;17:3003–5.
Lou K, Yao Y, Hoye AT, James MJ, Cornec AS, Hyde E, et al. Brain-penetrant, orally bioavailable microtubule-stabilizing small molecules are potential candidate therapeutics for Alzheimer’s disease and related tauopathies. J Med Chem. 2014;57:6116–27.
UCSF Chimera Home Page. http://www.cgl.ucsf.edu/chimera/. Accessed 20 Jan 2020.
Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010;31:455–61.
Castoldi M, Popov AV. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expr Purif. 2003;32:83–8.
Peloquin J, Komarova Y, Borisy G. Conjugation of fluorophores to tubulin. Nat Methods. 2005;2:299–303.
Yadava U, Gupta H, Roychoudhury M. Stabilization of microtubules by taxane diterpenoids: insight from docking and MD simulations. J Biol Phys. 2015;41:117–33.
Li Y, Han L, Liu Z, Wang R. Comparative assessment of scoring functions on an updated benchmark: 2. evaluation methods and general results. J Chem Inf Model. 2014;54:1717–36.
Srivastava HK, Chourasia M, Kumar D, Sastry GN. Comparison of computational methods to model DNA minor groove binders. J Chem Inf Model. 2011;51:558–71.
Rao S, He L, Chakravarty S, Ojima I, Orr GA, Horwitz SB. Characterization of the Taxol binding site on the microtubule. Identification of Arg282 in β-tubulin as the site of photoincorporation of a 7-benzophenone analogue of Taxol. J Biol Chem. 1999;274:37990–4.
Kumar N. Taxol-induced polymerization of purified tubulin. Mechanism of action. J Biol Chem. 1981;256:10435–41.
Kabir AMR, Wada S, Inoue D, Tamura Y, Kajihara T, Mayama H, et al. Formation of ring-shaped assembly of microtubules with a narrow size distribution at an air-buffer interface. Soft Matter. 2012;8:10863–7.
Rothwell SW, Grasser WA, Murphy DB. End-to-end annealing of microtubules in vitro. J Cell Biol 1986;102:619–27.
Prabhune M, Von Roden K, Rehfeldt F, Schmidt CF. Sulfo-SMCC prevents annealing of taxol-stabilized microtubules in vitro. PLoS ONE. 2016;11:e0161623.
Inoue D, Nitta T, Kabir AMR, Sada K, Gong JP, Konagaya A, et al. Sensing surface mechanical deformation using active probes driven by motor proteins. Nat Commun. 2016;7:12557.
Matsuda K, Kabir AMR, Akamatsu N, Saito A, Ishikawa S, Matsuyama T, et al. Artificial smooth muscle model composed of hierarchically ordered microtubule asters mediated by DNA origami nanostructures. Nano Lett. 2019;19:3933–8.
Acknowledgements
This work was financially supported by the Robot Technology Research and Development Project from New Energy and Industrial Technology Development Organization (NEDO), Japan, Grant-in-Aid for Scientific Research on Innovative Areas “Molecular Engine” (JP18H05423 and JP18H03673) and Grant-in-Aid for Young Scientists (A) to AK from the Japan Society for the Promotion of Science (JSPS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nasrin, S.R., Ishihara, T., Kabir, A.M.R. et al. Comparison of microtubules stabilized with the anticancer drugs cevipabulin and paclitaxel. Polym J 52, 969–976 (2020). https://doi.org/10.1038/s41428-020-0334-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-020-0334-9