Abstract
Background
Despite treatment with pancreatic enzyme replacement therapy (PERT), patients with cystic fibrosis (CF) can still suffer from fat malabsorption. A cause could be low intestinal pH disabling PERT. The aim of this study was to assess the association between faecal pH (as intestinal pH surrogate) and coefficient of fat absorption (CFA). Additionally, faecal free fatty acids (FFAs) were quantified to determine the amount of digested, but unabsorbed fat.
Methods
In a 24-h pilot study, CF patients followed a standardised diet with fixed PERT doses, corresponding to theoretical optimal doses determined by an in vitro digestion model. Study variables were faecal pH, fat and FFA excretion, CFA and transit time. Linear mixed regression models were applied to explore associations.
Results
In 43 patients, median (1st, 3rd quartile) faecal pH and CFA were 6.1% (5.8, 6.4) and 90% (84, 94), and they were positively associated (p < 0.001). An inverse relationship was found between faecal pH and total fat excretion (p < 0.01), as well as total FFA (p = 0.048). Higher faecal pH was associated with longer intestinal transit time (p = 0.049) and the use of proton pump inhibitors (p = 0.009).
Conclusions
Although the clinical significance of faecal pH is not fully defined, its usefulness as a surrogate biomarker for intestinal pH should be further explored.
Impact
-
Faecal pH is a physiological parameter that may be related to intestinal pH and may provide important physiopathological information on CF-related pancreatic insufficiency.
-
Faecal pH is correlated with fat absorption, and this may explain why pancreatic enzyme replacement therapy is not effective in all patients with malabsorption related to CF.
-
Use of proton pump inhibitors is associated to higher values of faecal pH.
-
Faecal pH could be used as a surrogate biomarker to routinely monitor the efficacy of pancreatic enzyme replacement therapy in clinical practice.
-
Strategies to increase intestinal pH in children with cystic fibrosis should be targeted.
Similar content being viewed by others
Introduction
Around 85% of paediatric patients with cystic fibrosis (CF) suffer from pancreatic insufficiency. The obstruction of the pancreatic duct precludes the release of pancreatic enzymes into the intestine, resulting in nutrient maldigestion and malabsorption.1
Pancreatic enzyme replacement therapy (PERT) consists of the exogenous administration of encapsulated pancreatic enzymes (amylase, protease and lipase) with every meal, leaving PERT as the standard of care to treat pancreatic insufficiency, and thereby to help prevent malnutrition.1,2 While digestion of carbohydrates and protein is generally satisfactory, digestion of fat can be compromised despite PERT.3,4 Pancreatic lipase requires an alkaline pH for optimal activity (it is inactive at pH lower than 5.5), which is altered in CF due to decreased secretion of sodium bicarbonate into the duodenum.4,5 The complete process of fat digestion (fat hydrolysis or lipolysis) implies the breakdown of triglyceride molecules into free fatty acids (FFAs)6 and their absorption in the intestine.7,8 If digestion is disturbed due to abnormally low pH and/or lack of pancreatic enzymes, triglycerides would neither be hydrolysed nor absorbed and would be excreted in the faeces as non-digested fat.6,9 On the other hand, it is possible that fat is hydrolysed into FFA, but not absorbed due to bile acid deficiency, resulting in the lack of micelle formation.10
In fact, previous studies have shown that small bowel pH in CF is positively associated with the efficacy of PERT.6 Lipid digestion was evaluated in several studies within the MyCyFAPP Project;11 first, an in vitro study determined the theoretical optimal doses (TODs) of enzymes needed for maximum lipolysis from different food products, in the CF-specific intestinal environment.9 The TODs were next evaluated in an in vivo pilot study: all patients adhered to the same standard 24-h diet and used the same dose of PERT (the TOD), to identify patient-specific factors that may influence lipolysis.12 This resulted in a median coefficient of fat absorption (CFA) of 90%,12 suggesting that adjustment of PERT on TOD was accurate enough for adequate dietary fat digestion and absorption. A subgroup of the study population could not reach the 90% clinical target; however, none of the patient characteristics that were considered could explain this result. Therefore, we hypothesised that a low small intestine pH may have caused insufficient PERT activity, leading to fat maldigestion and malabsorption, as previously reported in a small series of patients with CF.6 In addition, we hypothesised that assessing FFA excretion could be an indicator of the efficacy of the administered PERT dose, as their presence in faeces would indicate digestion was effective, but absorption was impaired.
To test this hypothesis, the faecal samples collected in the MyCyFAPP study were further assessed for pH and FFA, in addition to fat excretion quantification. Given the impossibility to assess intestinal pH in this series of patients, faecal pH was explored as a surrogate marker.
Therefore, the primary objective of the present study was to investigate the association between CFA and faecal pH. Secondarily, we aimed to explore total faecal FFA concentration as an additional biological indicator of the efficacy of the fat digestion process in patients with controlled dietary fat and PERT intake.
Patients, materials and methods
Subjects and study design
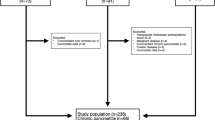
Study patients were regularly followed at five European CF Centres (Madrid, Valencia, Milan, Leuven and Rotterdam), with a confirmed CF diagnosis, pancreatic insufficiency, age between 2 and 18 years in stable gastrointestinal and respiratory conditions since at least 2 weeks before signing the informed consent. Acute infections, use of antibiotics, severe cholestasis and changes in the usual treatment during the 2 weeks before enrolment were considered as the exclusion criteria. Details about inclusion and exclusion criteria, as well as the study design, are explained in detail in a previous publication by our group.12 The Ethics Committees of all the participating centres approved the study protocol.
Patients were instructed to adhere to a standardised 24-h test diet with corresponding fixed PERT doses (based on the TODs, obtained during the in vitro studies).9 In this way, the influence of patient-specific characteristics on fat digestion could be evaluated. The diet consisted of five meals, starting with an afternoon snack (toasted bread with butter and jam) and dinner (ham and cheese sandwich) on the first day, and breakfast (milk with breakfast cereal), morning snack (yoghurt and orange juice) and lunch (pizza and dairy desert) on the second day. Portions could be adjusted according to age and individual preferences. PERT doses were strictly instructed per each meal, corresponding to the TOD. Encapsulated dyes (E-120, red carmine and E-132, indigo blue) were ingested at the start and at the end of the 24-h fixed diet in order to identify the faeces specifically pertaining to the study period.12
Clinical parameters obtained were age, gender, genotype, proton pump inhibitor (PPI) use, transit time, total faecal fat (and the corresponding CFA), total faecal FFA and faecal pH.
Faecal analyses
The faecal samples were frozen (−20 to −80 °C) and shipped from all the participating centres to the central lab in Instituto de Investigación Sanitaria La Fe (Valencia, Spain) for analysis. Since there is no well-described or generally accepted standardised method for homogenisation of faecal samples in the literature,13,14,15,16 the homogenisation of samples was carried out carefully after thawing at room temperature. The samples that were not dyed were discarded before homogenisation. Faeces were mixed with 750 W stirrers (Braun MQ735) until complete homogenisation was obtained (~5 min per sample, depending on the consistency and volume).
Total faecal fat
Total faecal fat (including both triglycerides and FFAs) was quantified on the homogenised faecal samples (10 g) with infrared spectroscopy, the gold standard to analyse fat in faeces.1 Random selected samples (n = 10) were used to evaluate the developed homogenisation protocol.
Coefficient of fat absorption
The CFA was calculated as the percentage of grams of fat excreted in the collected faeces relative to the grams of fat in the test diet.12,13,14,15,16,17 This parameter is considered equivalent to the lipolysis extent used in the in vitro setting. Transit time was calculated as the time between the ingestion of the colour markers and the moment the first dyed stool appeared.
FFAs in faeces
Aliquots from the homogenised faecal samples (5 g) were lyophilised and esterified. Then, the FFA profile was characterised by gas chromatography-mass spectrometry (GC-MS) technique.18
Faecal pH measurement
Aliquots from the homogenised samples (5 g) were thawed at room temperature and then mixed with 10 ml of deionized water and homogenised for 1–2 min using, firstly, a vortex and then using a horizontal shaker for 10 min (200 r.p.m.). Faecal pH was measured by directly inserting the glass electrode of the Accumet AE150 pH Benchtop-Meter (Fisher Scientific) into the homogenised faeces. Measurements were taken twice per sample. In addition, in a random selection of 20 samples, pH was measured in three different moments over a period of 24 h and repeated 1 month later to assess possible changes in the measurement over time.
Statistical analysis
A pilot study was conducted, provided that there was not an estimated effect or association to refer to given the lack of studies about the association between CFA and faecal pH. Therefore, an estimation of the statistical power was not required. Preparatory studies are designed to test the performance characteristics and capabilities of measures, procedures and operational strategies, and/or provide the means to evaluate aspects of novel approaches.19,20 Recommended sample sizes for pilot study ranged between 10 and 30 participants for pilot studies.21
A β-regression model was used to study the effect of faecal pH on CFA. Logarithmic transformation of faecal fat in stools and transit time was performed to approach a normal distribution with normalised extreme values. The association between faecal pH and the total amount of fat in stools (g) was studied by a univariate linear regression model, whereas the effect of faecal pH on total FFA (mg/g faeces), and the effect of FFA on CFA, was studied by means of a linear regression robust model. Finally, a linear regression multivariate robust model was established to study the association of age, transit time and use of PPI on faecal pH.
Analyses were carried out using the R software (version 3.5.1) and the libraries betareg (version 3.1-0), clickR (version 0.3.64) and MASS (version 7.3-49). A p value <0.05 was considered statistically significant.
Results
Patient characteristics and descriptive results
Forty-three children with CF were included. The study population characteristics were previously described by Calvo-Lerma et al.12 and are summarised in Table 1.
Association of faecal pH with CFA, and FFA excretion
As shown in Table 1, the median total amount of fat in faeces was 8.4 (4.8, 12.3) g, resulting in a median CFA of 90% with a small range between 1st and 3rd quartile (84, 94%). The total amount of FFAs was 2.3 (1.6, 3.6) mg/g of faeces. The median pH of the samples was 6.08 (5.78, 6.38), with a minimum of 5.26 and a maximum of 6.85.
Reproducibility of the faecal pH measurement was high, with a mean standard deviation of 0.02 when measuring the pH in three different points of the homogenised sample. No changes were detected in the pH measurement along a 24-h period. Test results were highly reproducible (coefficient of variation <15%) between two different aliquots of the same homogenised sample.
The faecal pH was significantly associated with the CFA (p < 0.001, R2 = 0.42) (Fig. 1a), with higher pH values relating to higher CFA. This result is confirmed by the significant inverse relationship between the pH and the total amount of fat in faeces (Fig. 1b), as the diet was fixed (p < 0.001, R2 = 0.38). This finding was also reinforced by the inverse association between faecal pH and the total amount of FFA (Fig. 1c) (p = 0.048, R2 = 0.35): the lower the pH, the higher the total amount of fat and FFA in faeces.
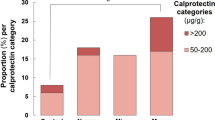
When considering patients on PPI therapy separately, median faecal pH values was 6.34 (6.09, 6.72), as compared to 5.91 (5.57, 6.31), in those not assuming this therapy (p < 0.01) (Fig. 2).
Faecal pH was positively and significantly associated with transit time (p = 0.049) and with the use of PPI (p = 0.009) in a multivariable analysis (Table 2). In contrast, neither the age of the patient nor any of the other study variables showed any association with faecal pH values.
In addition, a negative relationship was documented between CFA and FFA excretion (Fig. 3); low CFA being associated with high amount of faecal FFA (p < 0.001) suggests that lipolysis is occurring, but the FFAs were not being absorbed.
Discussion
In this study, we tried to further explore the mechanisms of fat absorption in CF. We have therefore characterised total fat and FFA excretion and pH in faecal samples of children with CF adhering to a standardised diet and using fixed doses of PERT, based on a CF-specific in vitro digestion model. Both faecal pH and FFA were used as an investigational tool. Faecal collection was very accurate, which was performed between colorimetric dyes, and this enabled us to measure transit time.
First, we found that faecal pH was positively associated with CFA, and inversely related with total FFA. In our previous study, we concluded that when using the in vitro predicted optimal dose of enzymes (TOD), CFA was not associated with any of the assessed patients’ characteristics (genotype, nutritional status, lung function, age), with the only exception of transit time.12 We concluded that other patients’ characteristics could have had an impact on CFA, particularly intestinal pH.12 Thus, the present findings on faecal pH may explain why some patients did not reach the 90% target CFA, supporting our research hypothesis: the association between faecal pH and CFA suggests that the TOD cannot achieve satisfactory levels of fat digestion in patients with a faecal pH lower than the pH range for lipase activity. Moreover, we found that higher CFA was related to lower FFA excretion, suggesting that in patients with satisfactory fat digestion, the absorption of delivered FFA must have been efficient, as low amounts were present in faeces. Thus, this finding also suggests that when the in vitro predicted dose of PERT was prescribed, fat was not only well digested but also well absorbed. Conversely, some patients with low CFA tended to have high FFA excretion, pointing at additional factors explaining malabsorption. However, none of these patients’ characteristics could explain this finding.
A previous study by our group has shown the strong influence of the intestinal pH on lipolysis under simulated in vitro gastrointestinal digestion.9,22,23 One of the few studies published on this topic showed that patients on tube feeding (n = 18) with low intestinal pH profiles were unable to reach satisfactory levels of fat digestion and absorption, despite high PERT doses.6 Compared to that study, our sample size was larger (n = 43), and patients were on a normal diet (rather than being on enteral nutrition); however, faecal pH was measured as a surrogate of intestinal pH (given the impossibility to assess actual intestinal pH at this point because of the invasive procedure required for its measurement). Robinson et al.,6 in contrast, performed a direct measurement of the intestinal pH. Both studies, however, have obtained the same finding of the association between intestinal pH and fat absorption. Also, the proportion of patients with a very low intestinal pH (<5.8) was similar in both studies (~20%). It should be considered that other mechanisms have proved to exert a relevant role in fat digestion and absorption. Malabsorption of bile acids occurs in patients with CF, and abnormal biliary secretion or intraluminal acidic precipitation of bile acids could contribute to steatorrhea.24 This effect has been also shown in the context of in vitro digestion studies, in which reduced bile salt concentration has repeatedly been associated with decreased extents of lipolysis.9,22,23
Possible determinants for faecal pH values were explored, finding that PPI use and longer transit time were associated with higher pH values. Studies evaluating the pH profile along the gastrointestinal tract in a healthy population by using the wireless motility capsule and radiotelemetry device support the main assumption that faecal pH may be considered as a surrogate for intestinal pH, as pH in the duodenum (lipid digestion) and rectum (before faecal deposition) was equivalent.10,24,25 In the case of CF, small intestinal pH during the first hour of small intestine digestion ranges between 5 and 7,24,26 in agreement with the pH range found in the faecal samples of our patients. Overall, the mean intestinal pH reported by Aburub et al.10 for healthy subjects was 6.2 and, in our cohort, a median value of 6.02 was obtained. Despite this difference could appear to be small, a decrease of 0.2 in pH in terms of lipase enzyme activity can imply a substantial reduction of fat digestion.27 In addition, this difference in pH becomes higher if one considers those patients not following PPI therapy, and this would be reflected in lower fat digestion.
In addition, we also found that patients taking PPI have higher faecal pH. Treatment with PPI reduces gastric acid secretion and leads to less acidic stomach environment during digestion.28 As a consequence, the gastric contents enter the duodenum with a more alkaline pH than the usual, thus modifying the intestinal conditions towards a normal environment, despite the limited pancreatic bicarbonate secretion in patients with CF.10,18
The strengths of this study include the accuracy of faecal collection using colorimetric markers. The samples are of unique value since, for the first time to our knowledge, they represent the product of the digestion and absorption of the same diet and the same PERT doses followed by a cohort of 43 patients with CF. It was therefore possible to assess differences in CFA only in terms of individual patients’ characteristics, avoiding confounding factors related to the diet or the variability in PERT. Another strength relies on the GC-MS technique used, the most accurate and state-of-the-art method to quantify FFAs.29
On the other hand, our assumption that faecal pH reflects the pH of the small intestine may be considered simplistic. In the colon, several reactions occur, including fermentation of unabsorbed or undigested nutrients (carbohydrates, protein) by specific bacteria conforming the microbiota. This results in the production of short-chain fatty acids, which may reduce the luminal pH. However, even if our patients could have different microbiota profiles, the production of short-chain fatty acids is highly dependent on the pattern of food intake,30 and all of them had the same standardised meals. Thus, the inclusion of microbiota assessment may be of interest in future studies. Another factor possibly modifying the pH in the colon is related to carbohydrates malabsorption,31 which however was unlikely to be present in our patients since normally it is associated to liquid stools32 (present in only one of our patients.12 In light of the significant association between faecal pH, total faecal fat and FFAs and CFA, along with the comparable range of pH as in previous series, we suggest that this indirect measurement could reflect at least in part the intestinal conditions. If confirmed by further studies assessing the correlation between faecal and intestinal pH, faecal pH may become a non-invasive biomarker of the intestinal pH and provide insight into the mechanism of malabsorption in CF and possibly other malabsorptive states.
The strong association and moderate correlation between faecal pH and CFA reinforces the previous data obtained by our research group, indicating that strategies aimed at increasing intestinal pH may result in better fat absorption. Thus, our results support the use of PPI therapy in addition to PERT in patients with low CFA but already taking high doses of PERT, as previously concluded by Robinson et al.6 However, the clinical value of using faecal pH to guide management requires further evaluation. It should be always kept in mind that long-term PPI treatment may lead to complications by suppressing the natural barrier of the gastric acid against pathogenic bacteria33 and it has been associated with an increase in the number of pulmonary exacerbation and hospitalisation.34
Another clinical application, although with possible limitations, would be measuring pH in faeces as a complementary tool to indirectly assess fat digestion and absorption. It is a simple, non-invasive and fast technique that only requires a pH metre, a common and not expensive equipment. This technique could possibly complement the 24-h faeces collection.
In conclusion, faecal pH is suggested as a possible factor explaining differences found in the CFA in a cohort of patients with CF that followed the same diet and had the same doses of PERT according to an in vitro evidence-based method. Strategies to increase the intestinal pH in CF are encouraged in order to enhance lipid digestion, being aware of the potential side effects. In addition, the use of faecal pH as a surrogate for fat malabsorption could be a fast and easy technique, which with pertinent additional validation could be used in clinical practice.
References
Turck, D. et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 35, 557–577 (2016).
Borowitz, D., Baker, R. D. & Stallings, V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J. Pediatr. Gastroenterol. Nutr. 35, 246–259 (2002).
Fieker., A., Philpott, J. & Armand, M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin. Exp. Gastroenterol. 4, 55 (2011).
Sitrin, M. D. Digestion and Absorption of Carbohydrates and Proteins in the Gastrointestinal System 137–158 (Springer, Dordrecht, 2014).
Gelfond, D. et al. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig. Dis. Sci. 58, 2275–2281 (2013).
Robinson, P. J. et al. Duodenal pH in cystic fibrosis and its relationship to fat malabsorption. Dig. Dis. Sci. 35, 1299–1304 (1990).
Hunter, J. E. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 36, 655–668 (2001).
Hernell, O., Staggers, J. E. & Carey, M. C. Physical–chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestionin healthy adult human beings. Biochemistry 29, 2041–2056 (1990).
Calvo-Lerma, J. et al. A first approach for an evidence-based in vitro method to adjust pancreatic enzyme replacement therapy in cystic fibrosis. PLoS ONE 14, e0212459 (2019).
Aburub, A. Comparison of pH and motility of the small intestine of healthy subjects and patients with symptomatic constipation using the wireless motility capsule. Int. J. Pharm. 544, 158–164 (2018).
Calvo-Lerma, J. et al. Innovative approach for self-management and social welfare of children with cystic fibrosis in Europe: development, validation and implementation of an mHealth tool (MyCyFAPP). Br. Med. J. Open. 7, e014931 (2017).
Calvo-Lerma, J. et al. Clinical validation of an evidence-based method to adjust pancreatic enzyme replacement therapy through a prospective interventional study in paediatric patients with cystitic fibrosis. PLoS ONE 14, e0213216 (2019).
Koumantakls, G. & Radciltf, F. J. Estimating fat in feces by near-infrared reflectance spectroscopy. Clin. Chem. 33, 502–506 (1987).
Rivero-Marcotegui, A. et al. Water, fat, nitrogen, and sugar content in feces: reference intervals in children. Clin. Chem. 44, 1540–1544 (1998).
Korpi-Steiner, N. L. et al. Comparative analysis of fecal fat quantitation via nuclear magnetic resonance spectroscopy (1H NMR) and gravimetry. Clin. Chim. Acta 400, 33–36 (2009).
Dorsey, J. et al. Fat malabsorption in cystic fibrosis: comparison of quantitative fat assay and a novel assay using fecal lauric/behenic acid. J. Pediatr. Gastroenterol. Nutr. 50, 441–446 (2010).
Proesmans, M. & De Boeck, K. Omeprazole, a proton pump inhibitor, improves residual steatorrhoea in cystic fibrosis patients treated with high dose pancreatic enzymes. Eur. J. Pediatr. 162, 760–763 (2003).
Paz-Yépez, C. et al. Influence of particle size and intestinal conditions on in vitro lipid and protein digestibility of walnuts and peanuts. Food Res. Int. 119, 951–959 (2019).
Moore, C. G. et al. Recommendations for planning pilot studies in clinical and translational sciences. Clin. Transl. Sci. 4, 332–337 (2011).
Fitzpatrick, J. J. & Kazer, M. W. Encyclopedia of Nursing Research 3rd edn, Vol. 440 (Springer, New York, 2011).
Isaac, S. & Michael, W. B. Handbook in Research and Evaluation (Educational and Industrial Testing Services, San Diego, 1995).
Asensio-Grau, A. et al. Effect of cooking methods and intestinal conditions on lipolysis, proteolysis and xanthophylls bioaccessibility of eggs. J. Funct. Foods 46, 579–586 (2018).
Asensio-Grau, A. et al. Fat digestibility in meat products: influence of food structure and gastrointestinal conditions. Int. J. Food Sci. Nutr. 70, 530–539 (2019).
Regan, P. T. et al. Reduced intraluminal bile acid concentrations and fat maldigestion in pancreatic insufficiency: correction by treatment. Gastroenterology 7, 285–289 (1979).
Fallingborg, J. et al. pH‐profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment. Phamacol. Ther. 3, 605–614 (1989).
Fallingborg, J. Intraluminal pH of the human gastrointestinal tract. Dan. Med Bull. 46, 183–196 (1999).
Calvo-Lerma, J. et al. In vitro digestion models to assess lipolysis: the impact of the simulated conditions for gastrointestinal pH, bile salts and digestion fluids. Food Res. Int. 125, 108511 (2019).
Kalantzi, L. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 23, 165–176 (2006).
Zelles, L. & Bai, Q. Y. Fractionation of fatty acids derived from soil lipids by solid phase extraction and their quantitative analysis by GC-MS. Soil Biol. Biochem. 25, 495–507 (1993).
Fiorentini, G. et al. Effect of lipid sources with different fatty acid profiles on intake, nutrient digestion and ruminal fermentation of feedlot nellore steers. Asian-Australas. J. Anim. Sci. 28, 1583 (2015).
Perman, J. A., Modler, S. & Olson, A. C. Role of pH in production of hydrogen from carbohydrates by colonic bacterial flora. Studies in vivo and in vitro. J. Clin. Invest. 67, 643–650 (1981).
Sellin, J. H. & Hart, R. Glucose malabsorption associated with rapid intestinal transit. Am. J. Gastroenterol. 87, 5 (1992).
Tran, T. M. D. et al. Effects of a proton-pump inhibitor in cystic fibrosis. Acta Pediatr. 87, 553–558 (1998).
Ayoub, F., Lascano, J. & Morelli, G. Proton pump inhibitor use is associated with an increased frequency of hospitalization in patients with cystic fibrosis. Gastroenterol. Res. 10, 288 (2017).
Acknowledgements
We acknowledge the support of the MyCyFAPP Project consortium. We especially thank the participation and the effort of the patients involved in the study and their families. This work was fully funded by the European Union and the Horizon 2020 Research and Innovation Framework Programme (PHC-26-2014 call Self management of health and disease: citizen engagement and mHealth) under grant number 643806.
Author information
Authors and Affiliations
Contributions
J.C.-L., M.R., A.A. and C.R.-K. designed the research. M.B., B.d.K., A.B., M.G. and E.M. collected the samples and the data. M.R., A.A.-G. and J.C.-L. analysed the data. V.F.-F. performed the statistical analyses. J.C.-L., M.R., M.B., B.d.K., C.C. and J.H wrote the paper. A.A., K.d.B., C.C., J.H. and C.R.-K. revised and corrected the paper. All authors approved the final version of the article, including the authorship list. J.C.-L. is the submission’s guarantor (i.e. the person who takes responsibility for the integrity of the work as a whole, from inception to published article).
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Patient consent
Patient consent form was required to participate in this clinical research study. All the participants gave written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Calvo-Lerma, J., Roca, M., Boon, M. et al. Association between faecal pH and fat absorption in children with cystic fibrosis on a controlled diet and enzyme supplements dose. Pediatr Res 89, 205–210 (2021). https://doi.org/10.1038/s41390-020-0860-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0860-3