Abstract
BACKGROUND
We recently identified 35 women with polycystic ovarian syndrome (PCOS) who exhibited features of micronodular adrenocortical hyperplasia. Steroid hormone analysis can be more accurate using state-of-the-art ultra-performance convergence chromatography-tandem mass spectrometry (UPC2-MS/MS). We hypothesized that UPC2-MS/MS may be used to better define hormonally this distinct subgroup of patients with PCOS.
METHODS
Plasma from PCOS patients (n = 35) and healthy volunteers (HVs, n = 19) who all received dexamethasone testing was analyzed. Samples were grouped per dexamethasone responses and followed by UPC2-MS/MS analysis. When insufficient, samples were pooled from patients with similar responses to allow quantification over the low end of the assay.
RESULTS
The C11-oxy C19 (11β-hydroxyandrostenedione, 11keto-androstenedione, 11β-hydroxytestosterone, 11keto-testosterone):C19 (androstenedione, testosterone) steroid ratio was decreased by 1.75-fold in PCOS patients compared to HVs. Downstream steroid metabolites 11β-hydroxyandrosterone and 11keto-androsterone were also measurable. The C11-oxy C21 steroids, 11-hydroxyprogesterone and 11keto-dihydroprogesterone levels, were 1.2- and 1.7-fold higher in PCOS patients compared to HVs, respectively.
CONCLUSIONS
We hypothesized that UPC2-MS/MS may accurately quantify steroids, in vivo, and identify novel metabolites in a subgroup of patients with PCOS and adrenal abnormalities. Indeed, it appears that adrenal C11-oxy steroids have the potential of being used diagnostically to identify younger women and adolescents with PCOS who also have some evidence of micronodular adrenocortical hyperplasia.
Impact
-
Adrenal C11-oxy steroids may be clinically important in identifying young patients with PCOS and adrenal abnormalities.
-
The steroids presented in our manuscript have not yet been considered in the clinical setting so far, and we believe that this study could represent a first focused step towards the characterization of a distinct subgroup of women with PCOS who may in fact be treated differently than the average patient with PCOS.
-
This paper can change the understanding of PCOS as one disorder: it is in fact a heterogeneous condition. In addition, for the subgroup of patients with PCOS associated with adrenocortical dysfunction, our paper provides novel hormonal markers that can be used diagnostically. Finally, the paper also adds to the basic pathophysiological understanding of adrenocortical-ovarian interactions in steroidogenesis of young women and adolescent girls with PCOS.
Similar content being viewed by others
Introduction
Polycystic ovarian syndrome (PCOS) is a complex and multifactorial disorder in which identification of subpopulations of patients with distinct phenotypes may lead to elucidation of its many contributing pathophysiological mechanisms.1 We recently identified a pattern of glucocorticoid secretion in a subset of young women with PCOS, which mimicked that of patients with micronodular adrenocortical hyperplasia (MAH). These patients had smaller adrenal volumes and higher steroid hormone secretion after receiving dexamethasone (DEX) compared to the group of PCOS patients with appropriate responses to DEX.2 These patients were selected for the investigation due to their higher circulating dehydroepiandrosterone sulfate (DHEAS) and other adrenal steroids.2,3
The role of adrenal steroid excess in PCOS has been debated for a number of years. There appears to be no significant difference in basal hormones or in DHEAS levels in response to adrenocorticotropic hormone (ACTH) stimulation between patients responding or not responding to DEX,4 as well as in dehydroepiandrosterone (DHEA), DHEAS, testosterone (T), and androstenedione (A4) levels in adrenal vein samples after ACTH stimulation.5 However, ACTH stimulation in women with PCOS has also been shown to result in increased levels of 17α-hydroxyprogesterone (17OHPROG), deoxycortisol, and urinary free cortisol (UFC).6,7 In our previous study,2 UFC was significantly higher in PCOS patients who were selected based on high adrenal androgen levels. Although urinary 17-hydroxycorticosteroids (17OHCS) and 17-ketocorticosteroids (17KS) overall were not significantly different from control subjects, within the PCOS group, UFC and/or 17OHCS were detected at or above the 75th percentile in the subset of women, which indicated inadequate DEX suppression. We subsequently termed these patients high responders (HRs) based on their adrenal steroid response to DEX.
The aforementioned studies clearly point to the complexity of steroidogenesis in PCOS and suggest that the C19 steroids may not be the only hormones contributing to the PCOS phenotype. Given that ACTH stimulation increased deoxycortisol and UFC levels together with high adrenal androgen levels, it is evident that the adrenal’s contribution to the steroid pool is more than only the C19 steroids generally considered.8 The adrenal C11-oxy androgens, which include 11β-hydroxyandrostenedione (11OHA4) and 11-ketotestosterone (11KT), were recently reported in patients with PCOS.910 These C11-oxy C19 steroids have also been detected in normal female control subjects shown both in adrenal vein samples and in the peripheral circulation,5,11 while we have reported their presence in prostate cancer (PCa) tissue and in circulation, both in normal and PCa patients.12,13 Furthermore, the C11-oxy C19 steroids are detected at higher levels than the C19 steroids, A4 and T, only when not taking DHEA levels into account, in both the normal state and various diseases.5,9,13,14
In the present study, we hypothesized that analyzing a comprehensive steroid hormone profile of the HR group against the remaining patients with PCOS, who we termed normal responders (NRs), and comparing them both to healthy volunteers (HVs), would lead to new diagnostic hormonal profile applicable to this subset of patients with PCOS. In addition, the information would potentially shed new light into the steroidogenic abnormalities associated with pathogenesis of PCOS in this specific group of patients with PCOS, a highly heterogeneous disorder.
Subjects and methods
Subjects
The patients in this study have been previously described in detail.2 In brief, women 16–29 years old with PCOS defined as biochemical hyperandrogenism with associated findings of either menstrual irregularity or polycystic ovaries on ultrasound were eligible for participation. Hyperandrogenism was diagnosed if any of the following androgens were above the normal reference range: T, A4, DHEA, and DHEAS. A polycystic ovary on ultrasound was defined as having either 12 follicles measuring 2–9 mm in diameter or an increased ovarian volume of 10 cm3 or greater. Amenorrhea was defined as the absence of bleeding for at least 3 months; oligomenorrhea was defined as bleeding that occurred at an interval >35 days. In order to participate in the study, patients were required to have had oligomenorrhea for at least 6 months prior to initial admission. Patients had to be off oral contraceptives or any other medications that alter steroidogenesis for at least 1 month prior to participating in the study. We also documented history of oligomenorrhea prior to initiation of oral contraceptives. We excluded patients with late-onset congenital adrenal hyperplasia (CAH), untreated thyroid dysfunction, hyperprolactinemia, primary ovarian failure, and menarche within 2 years prior to participation in the study.
HVs aged 18–29 years were also recruited; their medical history was obtained and physical examination was performed by a physician on all healthy controls, who were otherwise healthy besides their obesity. A diagnosis of PCOS was excluded in all HVs by clinical and biochemical evaluation.
Clinical protocol
All patients were evaluated under the clinical protocol 11-CH-0119 (Clinical Trials Number: NCT01313455) that was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board. This was a prospective case–control study. Informed consent was obtained from all HVs and adult patients; the patients’ parents for all pediatric patients and older children signed an assent.
Participants were admitted as inpatients at the Clinical Research Center (CRC) at NIH for a total of 8 full days. Each subject provided 24-h urine collections for a total of 6 days. This study was done in the inpatient setting, as previously described:2 day 1 was the baseline; during days 2 and 3 subjects received low-dose DEX (0.5 mg) per os every 6 h (LDDST), and during days 4 and 5 they received 2 mg per os every 6 h (HDDST). Final blood and urine collection were done on day 6. In the present study, we report plasma steroid analyses only since UFCs and urine 17OHCS and 17KS levels were previously reported.2
Steroid analyses
Plasma samples were thawed on ice and vortexed, after which 0.5 mL was aliquoted. Internal standards of testosterone-D2 (1.5 ng), cortisol-D4 (15 ng), progesterone-D9 (15 ng), 11β-hydroxyandrostenedione-D7 (15 ng), and androstenedione-D7 (15 ng) were prepared in deionized water and added to samples (total volume 100 μL). The samples were vortexed in 5 mL methyl tert-butyl ether (MTBE) for 26 min, and subsequently frozen at −80 °C. Thereafter, the organic phase was removed, dried under nitrogen, and the dried residues were dissolved in 150 μL of 50% methanol in water.15 Subsequent to UPC2-MS/MS (ultra-performance convergence chromatography-tandem mass spectrometry) analysis, samples were combined within the three groups according to the Liddle’s test (PCOS HRs, PCOS NRs, and HVs) to allow analyses of steroid metabolites present at concentrations below the limit of quantification (LOQ). Four samples were combined, evaporated to dryness under a stream of nitrogen, and the residue resuspended in 100 µL of 50% methanol in water for analyses. In each of the three groups, three sets of samples (n = 3; representing 12 serum samples) were prepared.
UPC2-MS/MS quantification of steroid metabolites
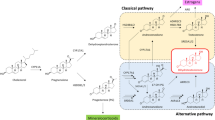
Steroids (A4, T, 5α-androstanedione (5αDIONE), dihydrotestosterone (DHT), androsterone (AN), 5α-androstan-3α,17β-diol (3αDIOL), dihydroprogesterone (DHPROG), DHEA, 17OHPROG, cortisone, cortisol, and corticosterone (CORT)) and MTBE were purchased from Sigma-Aldrich (St. Louis, MO, USA). 11OHA4, 11β- hydroxytestosterone (11OHT), 11keto-androstenedione (11KA4), 11KT, 11keto-DHT (11KDHT), 11β-hydroxyandrosterone (11OHAN), 11keto-AN (11KAN), 11keto-DHPROG (11KDHPROG), 5α-pregnan-17α-ol-3,20-dione (pdione), 5β-androstan-3β-ol-17-one (epietiocholanolone), 11keto-progesterone (11KPROG), 5β-androstan-3α-ol-11,17-dione (11K-etiocholanolone), 11β-hydroxyprogesterone (11βOHPROG), 5α-pregnan-3α,17α-diol-20-one (pdiol), 5β-androstan -3β,11β-diol-17-one (11OH-epietiocholanolone), 11α-hydroxyprogesterone (11αOHPROG), 21-deoxycortisol (21dF), and 5β-pregnan-3α,17α,20α-triol (pregnanetriol) were purchased from Steraloids Inc. (Newport, RI, USA). PROG was purchased from BDH Chemicals Ltd (Poole, UK) and 16α-hydroxyprogesterone (16OHPROG) was purchased from TRC (Toronto Research Chemicals, Toronto, Canada). Fetal bovine serum (FBS) superior was obtained from Biochrom GmbH (Berlin, Germany). All deuterated steroids were purchased from either Cambridge Isotope Laboratories Inc. (Andover, MA, USA) or CDN Isotopes Inc. (Quebec, Canada). See Supplementary Fig. 1 for a steroid metabolic pathway (classical, alternative, and backdoor) with the steroids included in this work.
Stock solutions of all steroids were dissolved in absolute ethanol (2 mg/mL) and stored at −20 °C. Standards ranging from 0.01 to 1000 ng/mL were extracted from FBS (500 μL) using the protocol described above. Linearity of the standard curves was assessed using the Runs test in GraphPad Prism and determined acceptable at r2 values (>0.9947) for all steroids.
The C19 and C21 steroids analyzed by ACQUITY UPC2®-MS/MS (Waters Corporation, Milford, USA; also termed ultra-high-performance supercritical fluid chromatography-tandem mass spectrometry) were separated using a Waters ACQUITY UPC2® BEH column (3 × 100 mm2, 1.7 µm particle size), with a 4.9 min linear elution gradient from 2% to 25% methanol used to separate the steroids (Supplementary Table 1). The mobile phase consisted of CO2 modified with methanol (+1% formic acid) in which steroids, in an injection volume of 2 µL, were eluted at a flow rate of 2 mL/min in a total run time of 6 min per sample. Mass transitions, limit of detection (LOD), and LOQ of all steroids are shown in Supplementary Table 1, indicating LODs and LOQs ranging from 0.01 to 10 ng/mL and from 0.01 to 20 ng/mL, respectively, the elution profile of the steroids are shown in Supplementary Fig. 2, and other MS-related settings have been previously published.12,13,16 Method validation has been published, including recovery, matrix effect (including the quantification of endogenous steroids in FBS), process efficiency, together with accuracy and precision,15 with the exception of CORT, which was quantified using the mass transition of 21dF in the present study. The method shows the recovery, matrix effect, and process efficiency of steroids isolated from a serum matrix to be within acceptable limits. Good accuracy, repeatability, and reproducibility were also shown, with acceptable % relative standard deviations (RSDs; <20%) obtained for all the steroids for both the accuracy (ranging from 1.5% to 20.0%) and precision (ranging from 1.6% to 19.9% tested over a 3-day period) parameters. Recovery ranged from 45.9% to 116.3%, matrix effect from −50.7% to 28.1%, and process efficiency from 36% to 118.7%.15 Data were collected and analyzed with the MassLynx 4.1 (Waters Corporation) software program.
Statistical analyses
Statistics were performed using an unpaired t test using the GraphPad Prism (version 6) software (GraphPad Software, San Diego, CA). One-way analysis of variance (ANOVA) and two-way ANOVA were used to test for possible differences between the three groups. Differences were considered statistically significant at P < 0.05. Principal component analysis (PCA) biplots were used to visually depict correlations between variables, as well as differences between the groups, and Spearman’s nonparametric tests were used to assess correlations between pairs of steroids. Furthermore, normality assumptions were reviewed by inspecting normal probability plots and were judged to be acceptable.
Results
Patients
The demographic and clinical characteristics of women with PCOS and HVs have been previously reported.2 In brief, the average age of PCOS patients was 20.4 ± 3.9, while HVs were 23.6 ± 2.6 years old. They had similar homeostasis model assessment of insulin resistance (3.5 ± 3.6 vs. 2.3 ± 1.5, PCOS vs. HVs, respectively). Thirteen of the women with PCOS (40%) had UFC and/or 17OHCS values at or above the 75th percentile (HR group). There were no statistically significant differences between this PCOS subsets vs. the remaining PCOS patients that we termed NRs (n = 22) in terms of their metabolic parameters, body mass index, and baseline values of T, DHEA, DHEAS, A4, and 17OH-pregnenolone.2 The group was distinguished, in addition, by the percentage change between baseline and day 5 for 17OHCS (HRs −29.5% ± 25.7% vs. NRs −55.4% ± 17.5%, P = 0.001), suggesting that the HRs not only had higher glucocorticoids but also suppressed less in response to DEX. Their adrenal volumes were also smaller, pointing to their overall similarity to the phenotype of patients with MAH.2
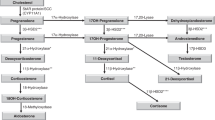
Analyses of the C19 steroids: classic androgens and C11-oxy androgens
Considering PCOS as a single group (Fig. 1), analysis of unconjugated C19 steroid levels showed that circulating DHEA, A4, and T were all significantly higher in PCOS patients (P < 0.001) than in the HV, and while 11KA4 levels were higher in the HV (P = 0.078), 11KT was the only C11-oxy androgen significantly higher in the PCOS group (P < 0.05). Data also show that C19 steroids, including DHEA, were present at higher levels than the C11-oxy C19 steroids, with T present at similar levels compared to 11OHT and 11KT combined.
DHEA, dehydroepiandrosterone; A4, androstenedione; T, testosterone; 11OHA4, 11β-hydroxyandrostenedione; 11OHT, 11β-hydroxytestosterone; 11KA4, 11keto-androstenedione; 11KT, 11keto-testosterone. Statistical significance was determined by multiple t tests of two-way ANOVA (*P < 0.05, ***P < 0.001, ****P < 0.0001).
DHEA, A4, and T levels in the PCOS women as HRs and NRs (Fig. 2a, c, d and Table 1) indicated no difference between these two groups regarding these C19 steroids with comparable A4:T ratios of 5.02 and 5.16, respectively, 1.2-fold greater than HV controls (4.42) (Fig. 2b). While 11OHA4, 11KA4, and 11OHT levels were also similar within the HR and NR groups, 11KT levels were higher in the NR group than in the HR group, and significantly higher (P = 0.01607) than in the HVs (Fig. 2e and Table 1). The levels of the downstream metabolite, 5αDIONE, the precursor to DHT in the alternative biosynthesis pathway, were similar in the HR and NR groups and comparable to the HVs (Table 1). Of interest is 3αDIOL (5α-androstane-3α, 17β-diol), the inactive metabolite of DHT, which was marginally lower in the HR group compared to the HV group, while in the NR group, 3αDIOL levels were lower, approaching significance (P = 0.06) (Fig. 2f).
PCOS women: HR, high responders, n = 15 (open black circles); NR, normal responders, n = 20 (open grey circles); and HV, healthy sex- and age-matched volunteers, n = 20 (closed black triangles). a Dehydroepiandrosterone (DHEA); b A4 (androstenedione): T (testosterone) ratio; c) A4; d T; e 11keto-testosterone (11KT); f 5α-androstane-3α,17β-diol (3αDIOL). Statistical significance was determined by one-way ANOVA and unpaired t tests (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, n.s. = not significant).
Analyses of the C11-oxy C21 steroids
Comparative analyses of the combined PCOS group and the HV group showed that only cortisol (P < 0.05) and cortisone (P < 0.001) were significantly higher and different from the controls (HVs) (Table 1). However, when analyzing the PCOS group as HR and NR groups, it was only in the HR group that cortisol levels were elevated (P < 0.05) when compared to HVs (Fig. 3a), while cortisone levels of both HR and NR groups were significantly increased (Fig. 3c) with cortisol:cortisone ratios, nevertheless comparable at 2.79 (NRs), 3.64 (HRs), and 3.46 (HVs) (Table 1). CORT levels were, however, higher in the HR group (P < 0.05) compared to the NR group and to the HVs (P < 0.01) (Fig. 3e). Analyses of PROG and its 16- and 17-hydroxylated metabolites (Fig. 3b, d, f) showed that these three steroids were lower in the NR group compared to the HR group (Table 1).
PCOS women: HR, high responders, n = 15 (open black circles); NRs, normal responders, n = 20 (open grey circles); and HV, healthy sex- and age-matched volunteers, n = 20 (closed black triangles). a Cortisol; b progesterone (PROG); c cortisone; d 17α-hydroxyprogesterone (17OHPROG); e corticosterone (CORT); f 16α-hydroxyprogesterone (16OHPROG). Statistical significance was determined by one-way ANOVA and unpaired t tests (*P < 0.05, **P < 0.01, ***P < 0.001).
We also investigated further downstream products of the C11-oxy C19 steroids, PROG, as well as the C11-oxy C21 steroids. Since these steroids were present below the LOQ, samples with similar steroid metabolite profiles were combined, therefore enabling our analysis of estimated levels. However, certain steroids remained too low to quantify and only those shown in Table 2 could be quantitated accurately. In addition, 11OH-epietiocholanolone and 11OHAN, as well as 11K-etiocholanolone and 11 KAN, could not be resolved chromatographically and were therefore quantified collectively as indicated in Table 2. These downstream metabolites were not statistically different when comparing the PCOS group to the HV group; however, 11OHAN and 11OH-epietiocholanolone, DHPROG, and 11KDHPROG were ~2-fold higher in the PCOS group (Table 2).
Steroids below the LOQ in the combined samples, detected in the three groups, were: DHT, 11KDHT, AN, epietiocholanone, 11KPROG, 11αOHPROG, pdione, pdiol, and pregnanetriol.
Relationships between steroids
We carried out a PCA to identify all relevant steroids other than those generally analyzed in the diagnosis of PCOS patients. Depicted in the PCA biplot is an overview of the statistical analyses of the HVs and the two PCOS group—a deviation from the cohort of control samples (Fig. 4, black circle) is indicative of a relationship between steroids correlated with the HR (grey circle) and NR (dashed circle) groups of PCOS patients. Both HR and NR groups lie askance from the HV group—indicating a shift in the relationship between certain steroids. As expected, increased A4 and DHEA steroid levels are positively correlated (Supplementary Figs. 3 and 4), reflecting the increased androgen production in hyperandrogenism characteristic of these PCOS patients. In addition, A4 and cortisone are visually correlated, as is 11OHA4 and cortisol (Supplementary Figs. 3 and 4). Cortisone, cortisol, CORT, and 16OHPROG are also positively correlated with each other in our PCOS group (Supplementary Figs. 3 and 4), suggesting that elevated levels of one would be associated with increased levels of the other. 11KT was also identified as a relevant steroid in PCOS and may be of importance when compared to the HV group, but more so in the NR group than in the HR group, reflecting the higher 11KT levels detected in our NR group (Fig. 2e). Interestingly, while PROG and 17OHPROG were strongly correlated (Supplementary Figs. 3 and 4), 11KT was negatively correlated with these C21 steroids (Fig. 4), reflecting the differences observed between the C21 and C19 steroid levels in the HR and NR groups within the PCOS group.
Discussion
The significance of adrenal androgen biosynthesis in the pathogenesis of PCOS has been the subject of intense investigation over the past half century. Our results, using a novel high-throughput UPC2-MS/MS method, point out that C11-oxy steroids have the potential of being used diagnostically to identify younger women and adolescents with PCOS who also have some evidence of MAH.
Our data show that circulating C19 steroid levels are significantly increased in the PCOS group with DHEA (±2.5-fold) and A4 (±2.1-fold) higher than in our healthy controls. While the ovary would also contribute to these steroids in circulation, the origin of the C11-oxy C19 steroids is attributed to the adrenals with the liver also potentially contributing towards 11OHT levels.17 11KT was significantly higher (±1.4-fold) in the PCOS group, and considering the C11-oxy C19 steroids collectively, these steroids were more abundant than A4 and T, when not taking DHEA into account. It should be noted that adrenal DHEA is also peripherally converted to A4 and T. In our investigation, we included the analyses of the downstream inactivated metabolites of DHT (3αDIOL and AN) and of 11OHA4 and 11KA4 (11KAN, 11OHAN, 11keto-etiocholanolone, and 11OH-epietiocholanolone). DHT was detected below the LOQ in both groups despite that A4 and T levels were higher in PCOS patients. AN was detected below the LOQ, while 3αDIOL was ±1.5-fold lower in PCOS compared to HVs. It may be that these metabolites, together with T and DHT, are conjugated and excreted efficiently in the PCOS group or that the inactivation by 3α-hydroxysteroid dehydrogenases (3αHSDs), such as AKR1C2 (3αHSD type 3) is less efficient in these patients. In addition, 5αDIONE levels were similar in the PCOS group compared to the control group suggesting the alternative pathway in the production of DHT may be comparable in both groups. Similar findings regarding the C11-oxy C19 steroids, in which 11KDHT was also detected below the LOQ, support the downstream metabolism of 11OHA4 and 11KA4 to their AN and etiocholanolone derivatives, via 5α- and 5β-reductases (SRD5As and AKR1D1, respectively) and AKR1C2.
The data show that the C11-hydroxy downstream metabolites were detected at higher levels than the C11-keto derivatives, in line with the higher 11OHA4 and lower 11KA4 precursor levels. Interestingly, even though 11OHA4 levels were not statistically different in PCOS patients, our PCA biplot indicated that the steroid was prominent in the HR group as is reflected in the shift towards elevated 11OHA4 levels in this group in comparison to the HV group, suggesting a potential role for this steroid in PCOS.
Further analysis of adrenocortical steroidogenesis between the two PCOS subgroups that we identified showed that while DHEA, T, and A4 levels were similar in the HR and NR groups, differences were apparent with 11KT levels being higher in the NR group, together with lower 3αDIOL, 11OHAN and 11OH-epietiocholanolone levels (1.5-fold), while 11KAN and 11keto-etiocholanolone were 4.4-fold higher. It would seem that the shunt towards androgen production and, in particular, adrenal androgen production as indicated by the C11-keto metabolites, is greater in the NR group in comparison to the HR group. Furthermore, our PCA biplot clearly showed that 11KT is negatively correlated with PROG and 17OHPROG, suggesting an increase in 11KT may lead to a decrease in PROG and 17OHPROG, as is reflected in their levels (Table 1). This is expected, as a shift in the production of androgens will decrease precursor steroids in the production of progesterone metabolites. Interestingly, 16OHPROG is positively correlated with the PCOS group (specifically the HR group), suggesting increases in this steroid could be a biomarker that may be studied in these patients in the future.
Considering the C21 steroids, cortisol and cortisone were significantly increased in the PCOS group, CORT levels were significantly higher in the HR group (2.24-fold) compared to the NR group. These data suggest that there is a greater steroid flux in the glucocorticoid and possibly the mineralocorticoid pathways of the HR group in comparison to the NR group. This was further supported by PROG levels being higher in the HR group (5.75-fold) compared to the NRs, as well as the levels of 17OHPROG (1.38-fold) and 16OHPROG (2-fold). We previously reported that recombinant cytochrome P450 17α-hydroxylase catalyzes 17OHPROG and 16OHPROG production at 4:1 with a 3.25:1 ratio shown in adult testicular microsomal preparations, and deviations from this ratio is associated with clinical conditions characteristic of adrenal disorders, such as Cushing’s syndrome, CAH, and in 21-hydroxylase-deficient (21OHD) patients.18,19,20 The increased 16OHPROG and 17OHPROG levels in our patients with PCOS clearly skewed the normal ratio, which is markedly smaller in the HR group (2.98) compared to the NR group (3.94). Furthermore, our in vitro studies recently identified downstream metabolites of 16OHPROG following the backdoor pathway, comparable to 17OHPROG,20,21 suggesting that these metabolites may have a role to play in PCOS. 17OHPROG and 21dF levels (median), although elevated in PCOS, lie within the normal range for these steroids in all of the groups22,23 ruling out other adrenal disorders such as CAH. 17OHPROG and 21dF are generally considered as markers in CAH characterized by 21OHD. Both 21dF and 11OHPROG were detected at higher levels, 2- and 1.3-fold, respectively, in the HR group, levels nevertheless were not within abnormal CAH ranges.
Contributing to circulating cortisol levels is the activation of cortisone; dysregulation of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) in PCOS has been implicated in the increased production of cortisol.24 Although our data show similar cortisone levels in both the HR and NR groups, we detected higher cortisol levels in the HR group; however, ratios did not differ significantly. It has been suggested that increased cortisol in PCOS patients leads to an upregulation of adrenal steroidogenesis resulting in C21 steroid production at the expense of C19 steroids,7 as is the case in the HR group in terms of the aforementioned steroids. Of note is 11βOHPROG, which was detected at higher levels in the HR group, with this steroid presenting as a potential substrate for peripheral 11βHSD type 2 (11βHSD2) and SRD5A enzymes yielding 11KPROG and subsequently 11KDHPROG, which we also detected to be present at higher levels (1.88-fold) in the HR group. In addition, the SRD5A reduced metabolite of PROG, and DHPROG was also higher in the HR group (1.3-fold higher), although these levels cannot be attributed solely to the adrenal production of PROG. While it has been reported that increased UFC together with that of adrenal androgen excretion is associated with increased peripheral conversion by SRD5A, not associated with body mass and insulin, SRD5A has also been reported to be upregulated in PCOS.25
The aforementioned C21 and C11-oxy C21 steroid metabolites, present at higher levels in the HR group, strongly suggest a greater steroid shunt towards the C21 steroids in this subset of PCOS women, also supported by data showing the peripheral conversion of PROG and 11OHPROG by SRD5A. We have shown that 21dF is converted to 11KDHT via 11KAN in the backdoor pathway26 together with 11OHPROG and 11KPROG also yielding 11KDHT via the same metabolic route.27 It is therefore possible that the C11-oxy C21 steroids, 21dF and 11OHPROG, and more specifically 11KDHPROG may add to the androgen pool via the backdoor pathway thus contributing to androgen excess in PCOS, more so in the HR group than in the NR group. Our data therefore suggests that in addition to measuring A4, T, and DHEA (and DHEAS) levels in PCOS patients, 11OHA4, 11KT, and perhaps DHPROG and 11KDHPROG should be included as biomarkers to analyze adrenal androgen excess in these patients. Furthermore, as CORT, together with cortisol and cortisone, also show a strong correlation in our PCOS patients, the overproduction of mineralocorticoids and glucocorticoids, including 16OHPROG, could also be monitored in these patients.
A limitation of the current study is that most of the steroid metabolites, necessary to fully characterize steroid panels in PCOS patients, are commercially unavailable and therefore the analyses of the complete C21 and C19 steroids, together with the C11-oxy C21 and C11-oxy C19, cannot be fully assessed in the metabolic pathways of patients. However, as this technology becomes cheaper and more available, studies like this can serve as guides for novel disease markers in PCOS and other disorders of aberrant steroidogenesis.
In conclusion, there appears to be indeed a subgroup of women with PCOS and abnormal adrenocortical steroidogenesis that may be detected by the measurement of specific novel biomarkers such as the C11-oxy C19 and C21 steroids. This study represents a small step towards the characterization of a distinct subgroup of women with PCOS who may in fact be suffering from an adrenocortical disorder. Whether this is also accompanied by a primary defect in ovarian steroidogenesis or their ovarian dysfunction is secondary to the abnormal adrenal steroids remains unknown. In any case, treatment of these women with PCOS may be fundamentally different in the future, as these patients could benefit from pharmacologic manipulation of their adrenocortical function to mitigate the signs and symptoms of PCOS.
References
Rosenfield, R. L. & Ehrmann, D. A. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr. Rev. 37, 467–520 (2016).
Gourgari, E. et al. Bilateral adrenal hyperplasia as a possible mechanism for hyperandrogenism in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 101, 3353–3360 (2016).
Crutchfield, C. A. et al. Comprehensive analysis of LC/MS data using pseudocolor plots. J. Am. Soc. Mass Spectrom. 24, 230–237 (2013).
Azziz, R., Black, V. Y., Knochenhauer, E. S., Hines, G. A. & Boots, L. R. Ovulation after glucocorticoid suppression of adrenal androgens in the polycystic ovary syndrome is not predicted by the basal dehydroepiandrosterone sulfate level. J. Clin. Endocrinol. Metab. 84, 946–950 (1999).
Rege, J. et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J. Clin. Endocrinol. Metab. 98, 1182–1188 (2013).
Maas, K. H. et al. Androgen responses to adrenocorticotropic hormone infusion among individual women with polycystic ovary syndrome. Fertil. Steril. 106, 1252–1257 (2016).
Yildiz, B. O. & Azziz, R. The adrenal and polycystic ovary syndrome. Rev. Endocr. Metab. Disord. 8, 331–342 (2007).
Kamrath, C., Wettstaedt, L., Boettcher, C., Hartmann, M. F. & Wudy, S. A. Androgen excess is due to elevated 11-oxygenated androgens in treated children with congenital adrenal hyperplasia. J. Steroid Biochem. Mol. Biol. 178, 221–228 (2018).
O’Reilly, M. W. et al. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 102, 840–848 (2017).
Davis, S. R., Turcu, A. F., Robinson, P. J. & Bell, R. J. Exogenous testosterone does not influence 11-oxygenated C19 steroid concentrations in healthy postmenopausal women. J. Endocr. Soc. 3, 670–677 (2019).
Turcu, A. F. & Auchus, R. J. Clinical significance of 11-oxygenated androgens. Curr. Opin. Endocrinol. Diabetes Obes. 24, 252–259 (2017).
du Toit, T. et al. Profiling adrenal 11beta-hydroxyandrostenedione metabolites in prostate cancer cells, tissue and plasma: UPC(2)-MS/MS quantification of 11beta-hydroxytestosterone, 11keto-testosterone and 11keto-dihydrotestosterone. J. Steroid Biochem. Mol. Biol. 166, 54–67 (2017).
du Toit, T. & Swart, A. C. Inefficient UGT-conjugation of adrenal 11beta-hydroxyandrostenedione metabolites highlights C11-oxy C19 steroids as the predominant androgens in prostate cancer. Mol. Cell. Endocrinol. 461, 265–276 (2018).
Turcu, A. F. et al. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur. J. Endocrinol. 174, 601–609 (2016).
du Toit, T., Stander, M. A. & Swart, A. C. A high-throughput UPC(2)-MS/MS method for the separation and quantification of C19 and C21 steroids and their C11-oxy steroid metabolites in the classical, alternative, backdoor and 11OHA4 steroid pathways. J. Chromatogr. B 1080, 71–81 (2018).
Bloem, L. M. et al. Advances in the analytical methodologies: profiling steroids in familiar pathways—challenging dogmas. J. Steroid Biochem. Mol. Biol. 153, 80–92 (2015).
Choi, M. H., Skipper, P. L., Wishnok, J. S. & Tannenbaum, S. R. Characterization of testosterone 11 beta-hydroxylation catalyzed by human liver microsomal cytochromes P450. Drug Metab. Dispos. 33, 714–718 (2005).
Swart, P., Swart, A. C., Waterman, M. R., Estabrook, R. W. & Mason, J. I. Progesterone 16 alpha-hydroxylase activity is catalyzed by human cytochrome P450 17 alpha-hydroxylase. J. Clin. Endocrinol. Metab. 77, 98–102 (1993).
Swart, A. C., Kolar, N. W., Lombard, N., Mason, J. I. & Swart, P. Baboon cytochrome P450 17alpha-hydroxylase/17,20-lyase (CYP17). Eur. J. Biochem. 269, 5608–5616 (2002).
van Rooyen, D. et al. The metabolic fate and receptor interaction of 16alpha-hydroxyprogesterone and its 5alpha-reduced metabolite, 16alpha-hydroxy-dihydroprogesterone. Mol. Cell Endocrinol. 441, 86–98 (2017).
van Rooyen, D., Gent, R., Barnard, L. & Swart, A. C. The in vitro metabolism of 11beta-hydroxyprogesterone and 11-ketoprogesterone to 11-ketodihydrotestosterone in the backdoor pathway. J. Steroid Biochem. Mol. Biol. 178, 203–212 (2018).
Tonetto-Fernandes, V. et al. Serum 21-deoxycortisol, 17-hydroxyprogesterone, and 11-deoxycortisol in classic congenital adrenal hyperplasia: clinical and hormonal correlations and identification of patients with 11beta-hydroxylase deficiency among a large group with alleged 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 91, 2179–2184 (2006).
Turcu, A. F. et al. Profiles of 21-carbon steroids in 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 100, 2283–2290 (2015).
Rodin, A., Thakkar, H., Taylor, N. & Clayton, R. Hyperandrogenism in polycystic ovary syndrome. Evidence of dysregulation of 11 beta-hydroxysteroid dehydrogenase. N. Engl. J. Med. 330, 460–465 (1994).
Vassiliadi, D. A. et al. Increased 5 alpha-reductase activity and adrenocortical drive in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 94, 3558–3566 (2009).
Barnard, L., Gent, R., van Rooyen, D. & Swart, A. C. Adrenal C11-oxy C21 steroids contribute to the C11-oxy C19 steroid pool via the backdoor pathway in the biosynthesis and metabolism of 21-deoxycortisol and 21-deoxycortisone. J. Steroid Biochem. Mol. Biol. 174, 86–95 (2017).
Gent, R., du Toit, T., Bloem, L. M. & Swart, A. C. The 11beta-hydroxysteroid dehydrogenase isoforms: pivotal catalytic activities yield potent C11-oxy C19 steroids with 11betaHSD2 favouring 11-ketotestosterone, 11-ketoandrostenedione and 11-ketoprogesterone biosynthesis. J. Steroid Biochem. Mol. Biol. 189, 116–126 (2019).
Acknowledgements
We thank Dr. Maya Lodish for assisting with the recruitment of patients and running the clinical protocol before its conclusion. We also thank Drs. Al Yergey (now deceased) and Peter Backlund of the NICHD Proteomics Core, who encouraged us to study novel steroid biomarkers in our patients and provided the first data that pointed to new steroid molecules in our patients. Many thanks to Drs. Elena Belyavskaya and Charalampos Lyssikatos for the collection, and Mrs. Maria de la Luz (Lucy) Sierra for handling successfully all the complexities of the sampling of this (and other) protocols. Finally, we are indebted to our HVs, our patients and their families who participated in this study, as well as the nursing and technical staff of the 1 and 5NW wards of the NIH-CRC; without them this study could never have been completed. This work was supported by National Research Foundation (IFR170125217588, CSUR160414162143); L’Oréal-UNESCO For Women in Science Sub-Saharan Africa Regional Fellowships, NIH-CRC Bench-to-Bedside Program and the NIH’s Office of Women’s Health; and in part by the NICHD Intramural Research Program, Bethesda, MD, USA.
Author information
Authors and Affiliations
Contributions
Substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data (A.C.S., T. du T., E.G., M.K., M.K., F.R.F., C.A.S.); drafting the article or revising it critically for important intellectual content (A.C.S., E.G., C.A.S.); final approval of the version to be published (A.C.S., C.A.S.).
Corresponding author
Ethics declarations
Competing interests
C.A.S. holds patent on the PRKAR1A, PDE11A, and GPR101 genes and/or their function and his laboratory has received research funding from Pfizer Inc. F.R.F. holds patent on the GPR101 gene and/or its function. The other authors have nothing to disclose.
Patient consent
Informed consent was obtained from all HVs and adult patients; the patients’ parents for all pediatric patients and older children signed an assent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Swart, A.C., du Toit, T., Gourgari, E. et al. Steroid hormone analysis of adolescents and young women with polycystic ovarian syndrome and adrenocortical dysfunction using UPC2-MS/MS. Pediatr Res 89, 118–126 (2021). https://doi.org/10.1038/s41390-020-0870-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0870-1