Abstract
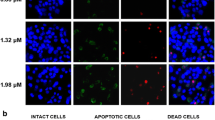
Cyclic dipeptides are increasingly gaining importance as considering its significant biological and pharmacological activities. This study was aimed to investigate the anticancer activity of a dipeptide Cyclo(-Pro-Tyr) (DP) identified from marine sponge Callyspongia fistularis symbiont Bacillus pumilus AMK1 and the underlying apoptotic mechanisms in the liver cancer HepG2 cell lines. MTT assay was done to demonstrate the cytotoxic effect of DP in HepG2 cells and mouse Fibroblast McCoy cells. Initially, apoptosis inducing activity of DP was identified using propidium iodide (PI) and acridine orange/ethidium bromide (AO/EB) dual staining, then it was confirmed by DNA fragmentation assay and western blotting analysis of apoptosis related markers Bax, Bcl-2, cytochrome c, caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP). Rhodamine 123 staining was performed to observe DP effects on the mitochondrial membrane potential (MMP) and DCFH-DA (Dichloro-dihydro-fluorescein diacetate) staining was done to measure the intracellular reactive oxygen species (ROS) levels. The MTT results revealed that DP initiated dose-dependent cytotoxicity in HepG2 cells, but no significant toxicity in mouse Fibroblast McCoy cells treated with DP at the specified concentrations. DP induced apoptosis, which is confirmed by the appearance of apoptotic bodies with PI and AO/EB dual staining, and DNA fragmentation. DP significantly elevated the Bax/Bcl-2 ratio, disrupted the mitochondrial membrane potential (MMP), enhanced cytochrome c release from mitochondria, increased caspase-3 activation, the cleavage of PARP and increased intracellular reactive oxygen species (ROS) levels. Besides this, DP successfully inhibited the phosphorylation of PI3K, AKT and increased PTEN expression. These results suggested DP might have anti-cancer effect by initiating apoptosis through mitochondrial dysfunction and downregulating PI3K/Akt signaling pathway in HepG2 cells with no toxicity effect on normal fibroblast cells. Therefore, DP may be developed as a potential alternative therapeutic agent for treating hepatocellular carcinoma.







Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics. CA Cancer J Clin 66(1):7–30. https://doi.org/10.3322/caac.21332
Daher S, Massarwa M, Benson AA, Khoury T (2018) Current and future treatment of hepatocellular carcinoma: an updated comprehensive review. J Clin Transl Hepatol 6(1):69–78. https://doi.org/10.14218/JCTH.2017.00031
Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C (2003) PI3K/Akt and apoptosis: size matters. Oncogene 22(56):8983–8998
Vitale FV, Romeo P, Luciani B, Raffaele M, Colina P, Ferraù F (2015) Mitomycin-based hepatic arterial infusion chemotherapy for solitary ampullary cancer liver metastasis: an unusual treatment for an uncommon disease. J Oncol Pharm Pract 21(5):396–399. https://doi.org/10.1177/1078155214531512
Wong RS (2011) Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 30:87. https://doi.org/10.1186/1756-9966-30-87
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. https://doi.org/10.1080/01926230701320337
Chang HY, Yang X (2000) Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev 64(4):821–846
Wang C, Youle RJ (2009) The role of mitochondria in apoptosis*. Annu Rev Genet 43:95–118. https://doi.org/10.1146/annurev-genet-102108-134850
Hata AN, Engelman JA, Faber AC (2015) The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov 5(5):475–487. https://doi.org/10.1158/2159-8290
Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G (2016) Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 8(4):603–619. https://doi.org/10.18632/aging.100934
Campbell KJ, Tait SWG (2018) Targeting BCL-2 regulated apoptosis in cancer. Open Biol. https://doi.org/10.1098/rsob.180002
Martelli AM, Evangelisti C, Chiarini F, McCubrey JA (2010) The phosphatidylinositol 3-kinase/Akt/mTOR signaling network as a therapeutic target in acute myelogenous leukemia patients. Oncotarget 1(2):89–103
Xie X, Tang B, Zhou J, Gao Q, Zhang P (2013) Inhibition of the PI3K/Akt pathway increases the chemosensitivity of gastric cancer to vincristine. Oncol Rep 30(2):773–782. https://doi.org/10.3892/or.2013.2520
Gardai SJ, Hildeman DA, Frankel SK, Whitlock BB, Frasch SC, Borregaard N, Marrack P, Bratton DL, Henson PM (2004) Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem 279(20):21085–21095
Kuo ML, Chuang SE, Lin MT, Yang SY (2001) The involvement of PI 3-K/Akt-dependent up-regulation of Mcl-1 in the prevention of apoptosis of Hep3B cells by interleukin-6. Oncogene 20(6):677–685
Shaw RJ, Cantley LC (2006) Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 441(7092):424–430
Rohde S, Nietzer S, Schupp PJ (2015) Prevalence and mechanisms of dynamic chemical defenses in tropical sponges. PLoS ONE 10(7):e0132236. https://doi.org/10.1371/journal.pone.0132236
Erba E, Bergamaschi D, Bassano L, Damia G, Ronzoni S, Faircloth GT, D'Incalci M (2001) Ecteinascidin-743 (ET-743), a natural marine compound, with a unique mechanism of action. Eur J Cancer 37(1):97–105
Kuznetsov G, Towle MJ, Cheng H, Kawamura T, TenDyke K, Liu D, Kishi Y, Yu MJ, Littlefield BA (2004) Induction of morphological and biochemical apoptosis following prolonged mitotic blockage by halichondrin B macrocyclic ketone analog E7389. Cancer Res 64(16):5760–5766
Xu Q, Huang KC, Tendyke K, Marsh J, Liu J, Qiu D, Littlefield BA, Nomoto K, Atasoylu O, Risatti CA, Sperry JB, Smith AB (2011) In vitro and in vivo anticancer activity of (+)-spongistatin 1. Anticancer Res 31(9):2773–2779
Piplani H, Rana C, Vaish V, Vaiphei K, Sanyal SN (2013) Dolastatin, along with Celecoxib, stimulates apoptosis by a mechanism involving oxidative stress, membrane potential change and PI3-K/AKT pathway down regulation. Biochim Biophys Acta 830(11):5142–5156. https://doi.org/10.1016/j.bbagen.2013.07.011
Janmaat ML, Rodriguez JA, Jimeno J, Kruyt FA, Giaccone G (2005) Kahalalide F induces necrosis-like cell death that involves depletion of ErbB3 and inhibition of Akt signaling. Mol Pharmacol 68(2):502–510
Huang R, Zhou X, Xu T, Yang X, Liu Y (2010) Diketopiperazines from marine organisms. Chem Biodivers 7(12):2809–2829. https://doi.org/10.1002/cbdv.200900211
Karanam G, Arumugam MK, Sirpu Natesh N (2019) Anticancer effect of marine sponge-associated Bacillus pumilus AMK1 derived dipeptide Cyclo (-Pro-Tyr) in human liver cancer cell line through apoptosis and G2/M phase arrest. Int J Pept Res Ther. https://doi.org/10.1007/s10989-019-09850-2
Sirpu Natesh N, Arumugam MK (2019) A novel apoptosis-inducing metabolite isolated from marine sponge symbiont Monascus sp. NMK7 attenuates cell proliferation, migration and ROS stress-mediated apoptosis in breast cancer cells. RSC Adv 9:5878–5890
Rahbar Saadat Y, Saeidi N, Zununi Vahed S, Barzegari A, Barar J (2015) An update to DNA ladder assay for apoptosis detection. Bioimpacts 5(1):25–28. https://doi.org/10.15171/bi.2015.01
Jiang J, Yu S, Jiang Z, Liang C, Yu W, Li J, Du X, Wang H, Gao X, Wang X (2014) N-acetyl-serotonin protects HepG2 cells from oxidative stress injury induced by hydrogen peroxide. Oxid Med Cell Longev. https://doi.org/10.1155/2014/310504
Rastogi RP, Singh SP, Häder DP, Sinha RP (2010) Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun 397(3):603–607. https://doi.org/10.1016/j.bbrc.2010.06.006
Das J, Sarkar A, Sil PC (2015) Hexavalent chromium induces apoptosis in human liver (HepG2) cells via redox imbalance. Toxicol Rep 2:600–608. https://doi.org/10.1016/j.toxrep.2015.03.013
Madankumar A, Jayakumar S, Gokuladhas K, Rajan B, Raghunandhakumar S, Asokkumar S, Devaki T (2013) Geraniol modulates tongue and hepatic phase I and phase II conjugation activities and may contribute directly to the chemopreventive activity against experimental oral carcinogenesis. Eur J Pharmacol 705(1–3):148–155. https://doi.org/10.1016/j.ejphar.2013.02.048
Nagabhishek SN, Kumar AM, Sambhavi B, Balakrishnan A, Katakia YT, Chatterjee S (2019) A marine sponge associated fungal metabolite monacolin X suppresses angiogenesis by down regulating VEGFR2 signaling. RSC Adv 9:26646–26667
Karmakar S, Banik NL, Ray SK (2007) Curcumin suppressed anti-apoptotic signals and activated cysteine proteases for apoptosis in human malignant glioblastoma U87MG cells. Neurochem Res 32(12):2103–2113
Al-Khayal K, Alafeefy A, Vaali-Mohammed MA, Mahmood A, Zubaidi A, Al-Obeed O, Khan Z, Abdulla M, Ahmad R (2017) Novel derivative of aminobenzenesulfonamide (3c) induces apoptosis in colorectal cancer cells through ROS generation and inhibits cell migration. BMC Cancer 17(1):4. https://doi.org/10.1186/s12885-016-3005-7
Lee Y, Phat C, Hong SC (2017) Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides 95:94–105. https://doi.org/10.1016/j.peptides.2017.06.002
Song J, Jeon JE, Won TH, Sim CJ, Oh DC, Oh KB, Shin J (2014) New cyclic cysteine bridged peptides from the sponge Suberites waedoensis. Mar Drugs 12(5):2760–2770. https://doi.org/10.3390/md12052760
Vázquez-Rivera D, González O, Guzmán-Rodríguez J, Díaz-Pérez AL, Ochoa-Zarzosa A, López-Bucio J, Campos-García J (2015) Cytotoxicity of cyclodipeptides from Pseudomonas aeruginosa PAO1 leads to apoptosis in human cancer cell lines. Biomed Res Int. https://doi.org/10.1155/2015/197608
Pfeffer CM, Singh ATK (2018) Apoptosis: a target for anticancer therapy. Int J Mol Sci. https://doi.org/10.3390/ijms19020448
Parrish AB, Freel CD, Kornbluth S (2013) Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a008672
Roy S, Bayly CI, Gareau Y, Houtzager VM, Kargman S, Keen SL, Rowland K, Seiden IM, Thornberry NA, Nicholson DW (2001) Maintenance of caspase-3 proenzyme dormancy by an intrinsic "safety catch" regulatory tripeptide. Proc Natl Acad Sci USA 98(11):6132–6137
Jiang X, Kim HE, Shu H, Zhao Y, Zhang H, Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S, Wang X (2003) Distinctive roles of PHAP proteins and prothymosin-alpha in a death regulatory pathway. Science 299(5604):223–226
Fischer U, Schulze-Osthoff K (2005) New approaches and therapeutics targeting apoptosis in disease. Pharmacol Rev 57(2):187–215
Reed JC (2000) Mechanisms of apoptosis. Am J Pathol 157(5):1415–1430. https://doi.org/10.1016/S0002-9440(10)64779-7
Ryan L, O'Callaghan YC, O'Brien NM (2004) Generation of an oxidative stress precedes caspase activation during 7beta-hydroxycholesterol-induced apoptosis in U937 cells. J Biochem Mol Toxicol 18(1):50–59
Tsujimoto Y (1998) Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells 3(11):697–707
Circu ML, Aw TY (2010) Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med 48(6):749–762. https://doi.org/10.1016/j.freeradbiomed.2009.12.022
Shi X, Wang J, Lei Y, Cong C, Tan D, Zhou X (2019) Research progress on the PI3K/AKT signaling pathway in gynecological cancer (Review). Mol Med Rep 19(6):4529–4535. https://doi.org/10.3892/mmr.2019.10121
Yuan TL, Cantley LC (2008) PI3K pathway alterations in cancer: variations on a theme. Oncogene 27(41):5497–5510. https://doi.org/10.1038/onc.2008.245
Liu Y, Xia XC, Meng LY, Wang Y, Li YM (2019) Alisol B 23-acetate inhibits the viability and induces apoptosis of non-small cell lung cancer cells via PI3K/AKT/mTOR signal pathway. Mol Med Rep 20(2):1187–1195. https://doi.org/10.3892/mmr.2019.10355
Yang J, Pi C, Wang G (2018) Inhibition of PI3K/Akt/mTOR pathway by apigenin induces apoptosis and autophagy in hepatocellular carcinoma cells. Biomed Pharmacother 103:699–707. https://doi.org/10.1016/j.biopha.2018.04.072
Yuan L, Wang J, Xiao H, Xiao C, Wang Y, Liu X (2012) Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol Appl Pharmacol 265(1):83–92. https://doi.org/10.1016/j.taap.2012.09.022
Hernández-Padilla L, Vázquez-Rivera D, Sánchez-Briones LA, Díaz-Pérez AL, Moreno-Rodríguez J, Moreno-Eutimio MA, Meza-Carmen V, Cruz H-D, Campos-García J (2017) The Antiproliferative effect of cyclodipeptides from Pseudomonas aeruginosa PAO1 on HeLa cells involves inhibition of phosphorylation of Akt and S6k kinases. Molecules 22:1024
Acknowledgements
The corresponding author is grateful to the grant sanctioned by Aquaculture and Marine biotechnology, Department of Biotechnology, Govt of India under research Grant (SAN No. 102/IFD/SAN/754/2016-2017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karanam, G., Arumugam, M. Reactive oxygen species generation and mitochondrial dysfunction for the initiation of apoptotic cell death in human hepatocellular carcinoma HepG2 cells by a cyclic dipeptide Cyclo(-Pro-Tyr). Mol Biol Rep 47, 3347–3359 (2020). https://doi.org/10.1007/s11033-020-05407-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05407-5