Abstract
Background
Wilms tumor (WT) with an inferior Vena cava (IVC) malignant thrombus comprises 4–10% of all WT cases.
Methods
This retrospective analysis included 51 pediatric patients presenting at Children Cancer Hospital Egypt-57357 from July 2007 to December 2016 with the diagnosis of WT with malignant IVC thrombus.
Results
Median age at presentation = 4.4 years and 28 cases (55%) were females. Twenty-five patients (49%) were metastatic and 4 patients (7.8%) had bilateral disease. Forty-seven cases (92.2%) had favorable histology with no evidence of anaplasia. Level of thrombus extension at presentation was classified as infra-hepatic, retro-hepatic, supra-hepatic and intra-cardiac in 33, 9, 6 and 3 patients, respectively. Fifty patients started neoadjuvant chemotherapy (CTH) with 16 patients showing complete resolution of thrombus after 6 weeks of CTH. None of the patients developed thrombus progression after neoadjuvant CTH; one patient had stationary intra-cardiac thrombus, while remaining patients showed partial regression of their thrombus and had nephrectomy with en-bloc thrombectomy. The mean cranio-caudal dimension of IVC thrombi at initial presentation was 6.5 cm, and 3.6 cm post 6th week of CTH. The 5-year OS and EFS were 75.9% and 71.1%, respectively. There was no significant correlation of initial levels of thrombus extension with survival.
Conclusion
Neoadjuvant chemotherapy followed by radical nephrectomy with en-bloc thrombectomy and radiotherapy seems a successful approach for management of patients with WT and IVC tumor thrombus. Measurement of the cranio-caudal dimension of thrombus and its response to treatment should be considered in the surgical planning.
Similar content being viewed by others
Introduction
Wilms tumor (WT) is the commonest renal tumor of childhood accounting for 6% of childhood cancer [1]. The prognosis for children with WT has improved dramatically over the last few decades as a result of coordinated trials and multi-modal therapy. WT is associated with an inferior Vena caval (IVC) thrombus in 4–10% of cases [2].
Many factors affect the management of WT with IVC thrombus including the extent of thrombus, tumor histology, stage, and its response to chemotherapy (CTH) [3]. Upfront nephrectomy procedure or neoadjuvant CTH with delayed surgery are valid options for management of retro-hepatic IVC thrombus, whereas neoadjuvant CTH is the primary approach in case of higher thrombus level [4].
We aim in this retrospective study to describe the characteristics of the largest cohort of WT patients with IVC thrombus reported from a single center up till now, and to discuss their management approach and survival outcome.
Methods
This study was approved by the Scientific and Medical Advisory Committee (SMAC) and the Institutional Review Board (IRB) of the Children Cancer Hospital Egypt-57357 (CCHE-57357). Data were collected retrospectively from medical records of patients diagnosed with WT at CCHE-57357 between June 2007 and December 2016. Collected data included demographic data, radiological studies, tumor stage, level of thrombus extension, CTH received, operative details, histopathologic diagnosis and survival outcomes. All patients with suspected WT had computerized tomography (CT) scans as a part of their initial work-up. Patients with suspected thrombus extension into IVC that was not clearly visualized on CT scans had a complementary Doppler US or abdominal magnetic resonance imaging (MRI). All patients with radiologically detected IVC thrombus were included. All radiographic exams were reviewed by an expert renal tumors radiologist to confirm diagnosis and extent of tumor thrombus.
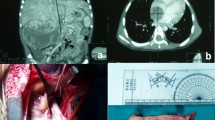
Patients were treated according to CCHE_RenTUL#7-v2-2012 protocol (Supplementary table S1) adopted from Children’s Oncology Group (COG) protocol AREN0532. Ultrasound-guided true-cut biopsy was performed to confirm diagnosis in patients with unilateral tumors planned for delayed surgical resection. Neoadjuvant CTH consisted of 6–12 weeks of 3-drug regimen containing Vincristine, Dactinomycin and Doxorubicin. Operative details included the surgical maneuver used with primary tumor, cavotomy, vascular thrombus excision, number of lymph nodes excised, capsular invasion and tumor spillage (Fig. 1). After nephrectomy, patients received radiotherapy over 5–7 days. Treatment continued for up to 25 weeks.
Surgical maneuver used for nephrectomy with en-bloc thrombectomy. During the nephrectomy procedure, the kidney is approached first and freed from all the attachments except for the involved renal vein. a The Vena cava is completely freed and controlled above and below the thrombus along with any tributaries. b The Vena cava is opened longitudinally and the thrombus is excised under vision and en-bloc without fragmentation or spillage into the abdominal cavity. c The thrombus is excised en-bloc altogether with the renal mass. In all patients of the study, Vena cava thrombus was resected completely en-bloc without the use of cell savers
For analysis purposes, the anatomical level of IVC thrombus in this study is classified as (1) infra-hepatic; thrombus extending into IVC below the edge of the liver, (2) retro-hepatic; thrombus extending into IVC above the edge of the liver but below the hepatic vein, and (3) supra-hepatic; thrombus extending above the level of hepatic vein, including intra-cardiac thrombi.
Radiological response of tumor thrombus is described in terms of average reduction in the thrombus cranio-caudal dimension (CCD) and in comparison to initial thrombus level in terms of complete thrombus resolution (CTR) where thrombus is no longer radiologically detected, partial thrombus regression (PTR) where thrombus regresses to a lower anatomical level, and stationary thrombus (ST) where thrombus remains within the same anatomical level regardless of regression in size or thickness.
Statistical analysis was done using SPSS software version 20.0 (IBM Corp., New York, NY, USA). Kaplan–Meier plots were drawn for event-free survival (EFS) and overall survival (OS), and comparison between groups were made by log rank test. EFS time was calculated from diagnosis by biopsy until first occurrence of relapse, progressive disease, death from treatment-related toxicity, or until last contact if no event occurred. OS was defined as the time from diagnosis to death from any cause. The nominal significance level was set at 0.05. Kruskal–Wallis and Mann–Whitney U tests were used when data were not normally distributed and one-way ANOVA test was used for normally distributed data.
To compare our results with the literature, we performed an electronic search of PubMed and Google Scholar databases, including papers published between January 1990 and August 2018 (Supplementary table S2).
Results
Fifty-one cases of WT with radiologically confirmed IVC thrombi accounted for 7.6% of all patients (n = 671) diagnosed with WT at our center in the same period of 9.5 years. Median age at diagnosis was 4.42 years (range 0.34–14), and 23 patients (45%) were males with right-sided tumors being more common (n = 29, 61.7% of unilateral cases). At time of diagnosis (nephrectomy or biopsy), 47 cases had favorable histology WT with no evidence of anaplasia (92.2%). According to COG staging system, 43.1% of patients (n = 22) were stage III, while 49% of them (n = 25) were stage IV and 7.8% (n = 4) were stage V. The commonest site for metastasis was the lungs (n = 22/25, 88%). Initial thrombus level was radiologically determined as infra-hepatic, retro-hepatic, supra-hepatic and intra-cardiac in 33, 9, 6 and 3 patients, respectively (Table 1). Two patients were excluded later in the analysis; one for incomplete response data, and another one developed pulmonary metastasis after 4 weeks of CTH and was treated off-protocol.
Of 49 evaluable cases, one patient—with initial infra-hepatic thrombus—underwent upfront nephrectomy with total thrombectomy, while all remaining patients started neoadjuvant CTH. After 6 weeks of CTH, no thrombus progression was observed in all patients. The thrombus response to neoadjuvant CTH was significantly associated with the initial anatomical level of IVC thrombi (P value < 0.001). CTR was seen in 16/48 cases (33.3%), all of whom had an infra-hepatic thrombus level initially. Remaining patients with initial infra-hepatic thrombus level (n = 16/32, 50%) showed regression in thrombus size (Fig. 2). There was no significant association of thrombus response with gender, tumor laterality, or initial tumor stage.
Retro-hepatic IVC thrombus was seen in 8 cases initially; 2 cases showed PTR after 6 weeks of CTH, while 6 cases showed ST level. All cases had nephrectomy with total thrombectomy by week 6 (n = 6) and week 12 (n = 2). Five patients had supra-hepatic extension of thrombus; 4 patients showed PTR after week 6, and the remaining patient had a bilateral WT with ST level that was completely resected post week 12 of CTH. Intra-cardiac thrombus was seen in 3 patients; one patient had a ST level after 12 weeks of CTH precluding its resection as no cardiac bypass could be performed, and the whole thrombus was irradiated post-operatively. The other two patients had PTR to retro-hepatic level and were amenable for resection by week 6 and week 12, respectively (Fig. 2).
The mean CCD of thrombi was 6.5 cm in 42 evaluable patients at presentation (range 1.5–22.5 cm), and 3.6 cm in 39 evaluable patients post week 6 (range 0–16 cm). Out of 39 patients, 21 patients (53.8%) had > 50% reduction in CCD of their thrombi. The median percentage of reduction in CCD post week 6 was 73.3%, 37.4% and 43.8% for infra-hepatic, retro-hepatic and supra-hepatic thrombi, respectively, with borderline statistical significance (P value = 0.05).
The median volume of tumor masses for 46 cases with available volume data was 782 cm3 at initial presentation and 167.6 cm3 post 6 weeks of CTH with a median percentage change of 79%. There was no significant correlation between tumor volume at presentation and anatomical level of thrombus (P value = 0.655), or with thrombus response after week 6 in terms of CTR, PTR, ST (P value = 0.091). Similarly, the percentage reduction in tumor volume did not significantly correlate with initial level of thrombi or with their response post 6 weeks of CTH (P value = 0.797). There was no significant difference in median tumor volume at presentation between our cohort and cases with local stage-III FHWT without IVC involvement (median volume for 157 cases with available data was 733.2 cm3, P value = 0.713).
Pathologic examination of IVC thrombus in 31 cases who had thrombectomy following neoadjuvant CTH showed completely necrotic tumor in 11 cases (35.5%), 8 cases (25.8%) showed viable malignant thrombus and remaining 12 cases (38.7%) had mixed therapy effect with viable tumor. There was no significant correlation between viability of malignant thrombi and their initial anatomical level (P value = 1.0) or the response of IVC thrombus after neoadjuvant CTH in terms of PTR or ST (P value = 1.0) or in terms of percentage change in CCD of thrombus (P value = 0.334 for 29 assessable cases).
For cases with stage IV FHWT with pulmonary-only metastasis (n = 22), there was no significant correlation between pulmonary response in terms of rapid complete response (RCR) vs slow incomplete response (SIR) and the response of IVC thrombus after 6 weeks of CTH in terms of CTR, PTR, ST (P value = 0.679) or in terms of percentage change in CCD of thrombus (P value = 0.81 for 18 assessable cases).
None of the included patients suffered from major surgical complications, pulmonary embolism or cardiac failure. The 5-year OS and EFS rates for the studied cohort are 75.9% and 71.1%, respectively. Neither OS nor EFS correlated significantly with initial anatomical levels of IVC thrombi (5-year OS for infra-hepatic, retro-hepatic and supra-hepatic was 81.1%, 56.3% and 72.9%, respectively, P value: 0.405 and 5-year EFS for infra-hepatic, retro-hepatic and supra-hepatic was 72.6%, 62.5% and 75%, respectively, P value: 0.769, Fig. 3). Similarly, there was no significant difference in EFS and OS for stage III patients with and without IVC thrombus (5-year OS of 85.6% and 86.2%, respectively, P value: 0.99 and 5-year EFS of 77.3% and 81.8%, respectively, P value: 0.65, Fig. 4).
Discussion
This study included 51 cases of WT with radiologically proven IVC thrombus, constituting the largest cohort of WT with IVC thrombus reported from a single center (Supplementary table S2). They represent 7.6% of our patients with WT, including 3 cases with intra-cardiac extension of thrombus. This is in agreement with other reports in the literature that found thrombosis involving the IVC in 4–10% of WT patients with 1–3% of them having right atrium involvement [5,6,7,8]. More than half of our cohort had the primary tumor in the right kidney, not matching to what was recently published by Al Diab et al., who observed left kidney involvement in (72%) of cases and they concluded with others that close proximity of the renal vein to the IVC does not seem to be a major factor in IVC thrombus formation [1, 9].
Despite different approaches, the COG and the International Society of Pediatric Oncology (SIOP) agree that WT with involvement of the IVC and right atrium benefit from preoperative chemotherapy [5, 7]. Neoadjuvant CTH is known to reduce the size of IVC thrombus, thus, facilitates surgery and lowers the risk of perioperative complications. Shamberger et al. reported a tumor thrombus regression in 39 of 49 patients after 8 weeks of neoadjuvant CTH [7]. In our cohort, one-third of cases had CTR in response to neoadjuvant CTH after 6 weeks and the majority of remaining cases regressed in size and extent of their thrombi. All patients with initial infra-hepatic thrombus showed marked regression in response to neoadjuvant CTH and half of them achieved CTR after 6 weeks of CTH. Similarly, Al Diab et al. reported complete resolution in four out of five patients (80%) with infra-hepatic thrombus [1]. Stationary level of thrombus after 6 weeks of CTH was observed in 75% and 20% of cases with retro-hepatic and supra-hepatic thrombi, respectively. Yet, this did not preclude successful thrombectomy, being achieved in all cases after a maximum of 12 weeks of CTH. This highlights the effect of neoadjuvant CTH on malignant thrombi in terms of reducing their thickness and loosening their adherence to caval wall in addition to lowering their anatomical level, all of which facilitates their surgical removal. The discrepancy in response between retro-hepatic and supra-hepatic thrombi might be attributed to the longer hepatic span causing many retro-hepatic thrombi to be labeled as having stationary level despite actual reduction in size and extent. Indeed, the median percentage of reduction in CCD were comparable for retro-hepatic and supra-hepatic thrombi by 6th week of CTH.
In our cohort, three cases presented with intra-cardiac thrombus; one case had a ST that precluded thrombectomy since cardiac bypass could not be done and post-operative RTH was given to the residual thrombus, while the other two had PTR and were surgically removed by week 6 and week 12, respectively. Interpreting the impact of neoadjuvant CTH on intra-cardiac thrombus in our cohort is limited by the small number of patients, but in general, the benefit of neoadjuvant CTH extends beyond down-leveling the thrombus to eliminating the need for cardiopulmonary bypass as noted by other studies [5, 7]. Similar to another report describing the response of retro-hepatic Vena cava thrombus to neoadjuvant CTH [2], none of our patients with retro-hepatic or supra-hepatic thrombi showed CTR by 6 or 12 weeks of CTH. Interestingly, another study showed complete resolution of an intra-atrial thrombus in a patient submitted to preoperative chemotherapy [10]. Other reports found no significant change in supra-hepatic thrombi after neoadjuvant CTH with tumor thrombus regression being noted in only 10–13% of cases [7, 11, 12]. However, it is noteworthy that—similar to our results—no child receiving neoadjuvant chemotherapy had a pulmonary embolism or major surgical complications [7, 11, 12]. This is very important to consider when deciding on the best approach to manage such patients. It is reasonable to assume that neoadjuvant CTH approach carries no additional risks of pulmonary embolism or other surgical complications.
There was no significant correlation between pulmonary response in patients with stage IV FHWT and the response of their IVC thrombi. Similarly, there was no correlation between the percentage reduction in tumor volume and the response of IVC thrombi. This could be attributed to the presence of pure thrombotic component in some thrombi leading to discrepant responses to neoadjuvant CTH.
The OS and EFS for WT cases with and without IVC thrombus were comparable (Fig. 4) and the anatomical level of thrombus did not impact survival outcome. Similar results have been reported by Aspiazu et al. [13], where the outcome of WT cases with intravascular extension was not affected by the presence or location of intravascular tumor thrombus, but an increased frequency of surgical complications has been reported. Other reports found an excellent survival rate in patients with intravascular tumor extension who were treated on National Wilms Tumor Study (NWTS) and SIOP protocols; there was no significant difference in survival outcomes in comparison with patients without intravascular extension when patients were matched by stage and histologic type [2, 3, 12].
This study demonstrates the effect of CTH on the cranio-caudal extent of thrombus in particular. The shrinkage in CCD is what matters most when it comes to surgery; most thrombi get their CCD reduced by at least 50% in response to 6 weeks of therapy and are amenable to total thrombectomy during nephrectomy. We believe that additional courses of neoadjuvant CTH are only to be given if the upper limit of thrombus is still above the diaphragm, since further reduction in CCD will reflect largely on the surgical approach. Otherwise, the operative approach and risk are largely the same, and thus, local control should not be delayed. This provides a highly customized approach to the decision making and to assess the value of further chemotherapy versus going to surgery in thrombi not very well responding to CTH according to available facilities, i.e. surgical, anesthetic and supportive care.
Conclusions
Results of our study show high survival rates for patients with WT and IVC thrombus which are not different from patients of the same histology and stage without an IVC thrombus. This observation indicates that the management strategy adopted is very efficient and may negate the presence of an IVC thrombus as an indicator of less favorable outcomes. Neoadjuvant CTH and delayed surgery in WT with IVC thrombus should be guided by the clinical situation but always considered. It can be awaited that the CCD of thrombus will shrink and thus improving the surgical management of this cohort of patients to achieve local control.
References
Al Diab A, Hirmas N, Almousa A, Abu-hijlih R, Aljlouni F, Sultan I et al (2017) Inferior vena cava involvement in children with Wilms tumor. Pediatr Surg Int 33:569–573. https://doi.org/10.1007/s00383-016-4034-7
Bader MI, Abdelaal K, Rogers T, Arul SG (2013) A surgical approach to Wilms’ tumour with retrohepatic vena caval extension. Pediatr Surg Int 29:229–232. https://doi.org/10.1007/s00383-013-3263-2
Khozeimeh N, Sinha P, Dome JS, Guzzetta PC (2011) Strategy for management of retroperitoneal tumors with caval tumor thrombus. J Pediatr Surg 46:2065–2070. https://doi.org/10.1016/j.jpedsurg.2011.06.041
Ritchey ML, Othersen HB, de Lorimier AA, Kramer SA, Benson C, Kelalis PP (1990) Renal vein involvement with nephroblastoma: a report of the National Wilms’ Tumor Study-3. Eur Urol 17:139–144
Hadley GP, Sheik-Gafoor MH, Buckels NJ (2010) The management of nephroblastoma with cavo-atrial disease at presentation: experience from a developing country. Pediatr Surg Int 26:1169–1172. https://doi.org/10.1007/s00383-010-2667-5
Lall A, Pritchard-Jones K, Walker J, Hutton C, Stevens S, Azmy A et al (2006) Wilms’ tumor with intracaval thrombus in the UK Children’s Cancer Study Group UKW3 trial. J Pediatr Surg 41:382–387. https://doi.org/10.1016/j.jpedsurg.2005.11.016
Shamberger RC, Ritchey ML, Haase GM, Bergemann TL, Loechelt-Yoshioka T, Breslow NE et al (2001) Intravascular extension of Wilms tumor. Ann Surg 234:116–121
McMahon S, Carachi R (2014) Wilms’ tumor with intravascular extension: a review article. J Indian Assoc Pediatr Surg 19:195–200. https://doi.org/10.4103/0971-9261.141998
Akyüz C, Emir S, Büyükpamukçu N, Atahan L, Cağlar M, Kutluk T et al (2005) Cavoatrial tumor extension in children with wilms tumor: a retrospective review of 17 children in a single center. J Pediatr Hematol Oncol 27:267–269
Cristofani LM, Duarte RJ, Almeida MT, Odone Filho V, Maksoud JG, Srougi M (2007) Intracaval and intracardiac extension of Wilms’ tumor. The influence of preoperative chemotherapy on surgical morbidity. Int Braz J Urol 33:683–689 (discussion 689)
Loh A, Bishop M, Krasin M, Davidoff AM, Langham MR (2015) Long-term physiologic and oncologic outcomes of inferior vena cava thrombosis in pediatric malignant abdominal tumors. J Pediatr Surg 50:550–555. https://doi.org/10.1016/j.jpedsurg.2014.11.044
Ritchey ML, Kelalis PP, Haase GM, Shochat SJ, Green DM, D’Angio G (1993) Preoperative therapy for intracaval and atrial extension of Wilms tumor. Cancer 71:4104–4110
Aspiazu D, Fernandez-Pineda I, Cabello R, Ramirez G, Alvarez-Madrid A, De Agustin JC (2012) Surgical management of wilms tumor with intravascular extension: a single-institution experience. Pediatr Hematol Oncol 29:50–54. https://doi.org/10.3109/08880018.2011.642941
Acknowledgements
We are grateful to the patients and their families for sharing tumor material and information, and thus contributing to the research efforts to understand and find cures for pediatric Wilms tumor with IVC thrombus. We would like to thank the CCHE-57357 group for continuous support.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Elayadi, M., Hammad, M., Sallam, K. et al. Management and outcome of pediatric Wilms tumor with malignant inferior Vena cava thrombus: largest cohort of single-center experience. Int J Clin Oncol 25, 1425–1431 (2020). https://doi.org/10.1007/s10147-020-01667-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01667-0