New Phosphine Oxides as High Performance Near- UV Type I Photoinitiators of Radical Polymerization
Abstract
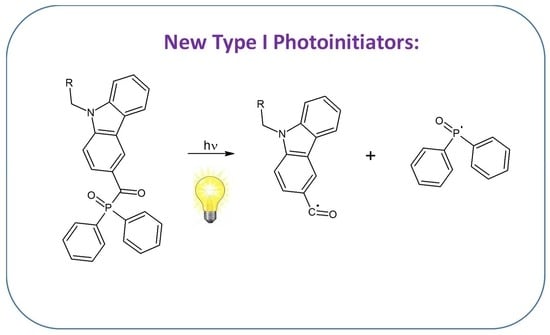
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Syntheses of the ADPO Derivatives
4.1.1. Synthesis of Compound CPO-1
4.1.2. Synthesis of Compound CPO-2
4.2. Other Chemicals
4.3. UV-Vis Spectroscopy
4.3.1. Spectrum Recording
4.3.2. Photolysis Experiment
4.4. Molecular Modeling
4.4.1. Geometries Optimization
4.4.2. UV-Vis Spectrum Calculation
4.5. RT-FTIR Spectroscopy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis: Scope, Reactivity and Efficiency; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Fouassier, J.P. Photoinitiation, Photopolymerization, Photocuring; Hanser: Münich, Germany, 1995. [Google Scholar]
- Fouassier, J.P.; Rabek, J.F. Radiation Curing in Polymer Science and Technology; Chapman & Hall: London, UK, 1993. [Google Scholar]
- Crivello, J.V.; Dietliker, K. Photoinitiators for Free Radical, Cationic and Anionic Photopolymerization, 2nd ed.; Surface Coatings Technology Series; Bradley, G., Ed.; Wiley: Chichester, UK, 1998. [Google Scholar]
- Dietliker, K.A. Compilation of Photoinitiators Commercially Available for UV Today; Sita Technology Ltd: London, UK, 2002. [Google Scholar]
- Belfied, K.D.; Crivello, J.V. Photoinitiated Polymerization; ACS Symp. Ser. 847; American Chemical Society: Washington, DC, USA, 2003. [Google Scholar]
- Allen, N.S. Photochemistry and Photophysics of Polymer Materials; Wiley: Hoboken, NJ, USA, 2010. [Google Scholar]
- Scranton, A.B.; Bowman, C.N.; Peiffer, R.W. Photopolymerization: Fundamentals and Applications; ACS Symp. Ser. 673; ACS: Washington, DC, USA, 1997. [Google Scholar]
- Kislyak, A.; Frisch, H.; Gernhardt, M.; Van Steenberge, P.H.M.; D’hooge, D.R.; Barner-Kowollik, C. Time-Dependent Differential and Integral Quantum Yields for Wavelength-Dependent [4+4] Photocycloadditions. Chem. Eur. J. 2020, 26, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.K.; Yagci, Y. Handbook of Vinyl Polymers; CRC Press: Bosa Roca, FL, USA, 2008. [Google Scholar]
- Ueno, K.; Oshikiri, T.; Sun, Q.; Shi, X.; Misawa, H. Solid-State Plasmonic Solar Cells. Chem. Rev. 2018, 118, 2955–2993. [Google Scholar] [CrossRef] [PubMed]
- Cambié, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef] [PubMed]
- Lalevée, J.; Fouassier, J.P. Dyes and Chromophores in Polymer Science; John Wiley&Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Gomurashvili, Z.; Hua, Y.; Crivello, J.V. Monomeric and Polymeric Carbazole Photosensitizers for Photoinitiated Cationic Polymerization. Macromol. Chem. Phys. 2001, 202, 2133–2141. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Garra, P.; Dumur, F.; Bui, T.T.; Goubard, F.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouasier, J.P.; et al. Novel Carbazole Skeleton-Based Photoinitiators for LED Polymerization and LED Projector 3D Printing. Molecules 2017, 22, 2143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, G.; Tuzun, A.; Yagci, Y. Thioxanthone-carbazole as a visible light photoinitiator for free radical polymerization. J. Polym. Sci. Part. A Polym. Chem. 2010, 48, 5120–5125. [Google Scholar] [CrossRef]
- Tunc, D.; Yagci, Y. Thioxanthone-ethylcarbazole as a soluble visible light photoinitiator for free radical and free radical promoted cationic polymerizations. Polym. Chem. 2011, 2, 2557–2563. [Google Scholar] [CrossRef]
- Karaca, N.; Balta, D.K.; Ocal, N.; Arsu, N. Mechanistic studies of thioxanthone–carbazole as a one-component type II photoinitiator. J. Lumin. 2014, 146, 424–429. [Google Scholar] [CrossRef]
- Nazir, R.; Danilevicius, P.; Gray, D.; Farsani, M.; Gryko, D.T. Push–Pull Acylo-Phosphine Oxides for Two-Photon-Induced Polymerization. Macromolecules 2013, 46, 7239–7244. [Google Scholar] [CrossRef]
- Sehnal, P.; Harper, K.; Rose, A.T.; Anderson, D.G.; Green, W.A.; Husár, B.; Griesser, M.; Liska, R. Novel Phosphine Oxide Photoinitiators. In Proceedings of the RadTech, Rosemont, IL, USA, 12–14 May 2014. [Google Scholar]
- Xie, C.; Wang, Z.; Liu, Y.; Song, L.; Liu, L.; Wang, Z.; Yu, Q. A novel acyl phosphine compound as difunctional photoinitiator for free radical polymerization. Prog. Org. Coat. 2019, 135, 34–40. [Google Scholar] [CrossRef]
- Dietlin, C.; Trinh, T.T.; Schweizer, S.; Graff, B.; Morlet-Savary, F.; Noirot, P.A.; Lalevée, J. Rational Design of Acyldiphenylphosphine Oxides as Photoinitiators of Radical Polymerization. Macromolecules 2019, 52, 7886–7893. [Google Scholar] [CrossRef]
- Wang, J.; Stanic, S.; Altun, A.A.; Schwentenwein, M.; Dietliker, K.; Jin, L.; Stampfl, J.; Baudis, S.; Liska, R.; Grutzmacher, H.A. A highly efficient waterborne photoinitiator for visible-light-induced three-dimensional printing of hydrogels. Chem. Commun. 2018, 54, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Eibel, A.; Schmallegger, M.; Zalibera, M.; Huber, A.; Buerkl, Y.; Gruetzmacher, H.; Gescheidt, G. Extending the Scope of Bis(acyl)phosphane Oxides: Additional Derivatives. Eur. J. Inorg. Chem. 2017, 2017, 2469–2478. [Google Scholar] [CrossRef]
- Marien, Y.W.; Van Steenberge, P.H.M.; Kockler, K.B.; Barner-Kowollik, C.; Reyniers, M.F.; Marina, G.B.; D’hoogeae, D.R. Estimating the photodissociation quantum yield from PLP-SEC peak heights. Polym. Chem. 2017, 8, 3124–3128. [Google Scholar] [CrossRef]
- Foresman, J.; Frish, E. Exploring Chemistry with Electronic Structure Methods; Gaussian Inc.: Pittsburg, PA, USA, 1996. [Google Scholar]
Sample Availability: Samples of the compounds CPO-1 and CPO-2 are available from the authors. |






| Molecular Modeling | Experimental Data | ||
|---|---|---|---|
| Molecules | λ max (nm) (Oscillator Strength) | ε395 nm (L/mol/cm) | ϕ |
| TPO-L | 383 (0.0005) | 131 | 0,3 [5] |
| BAPO | 384 (0.0006) | 664 | 0,6 [22] |
| CPO-1 | 384 (0.007) | 5600 | 0,7 |
| CPO-2 | 385 (0.007) | 3200 | 0,8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dietlin, C.; Trinh, T.T.; Schweizer, S.; Graff, B.; Morlet-Savary, F.; Noirot, P.-A.; Lalevée, J. New Phosphine Oxides as High Performance Near- UV Type I Photoinitiators of Radical Polymerization. Molecules 2020, 25, 1671. https://doi.org/10.3390/molecules25071671
Dietlin C, Trinh TT, Schweizer S, Graff B, Morlet-Savary F, Noirot P-A, Lalevée J. New Phosphine Oxides as High Performance Near- UV Type I Photoinitiators of Radical Polymerization. Molecules. 2020; 25(7):1671. https://doi.org/10.3390/molecules25071671
Chicago/Turabian StyleDietlin, Céline, Thanh Tam Trinh, Stéphane Schweizer, Bernadette Graff, Fabrice Morlet-Savary, Pierre-Antoine Noirot, and Jacques Lalevée. 2020. "New Phosphine Oxides as High Performance Near- UV Type I Photoinitiators of Radical Polymerization" Molecules 25, no. 7: 1671. https://doi.org/10.3390/molecules25071671