Directing 2D-Coordination Networks: Combined Effects of a Conformationally Flexible 3,2′:6′,3″-Terpyridine and Chain Length Variation in 4′-(4-n-Alkyloxyphenyl) Substituents
Abstract
:1. Introduction
2. Results and discussion
2.1. Synthesis and Characterization of Compound 3
2.2. Reactions of Compounds 2–6 with Co(NCS)2 and Bulk Material Characterization
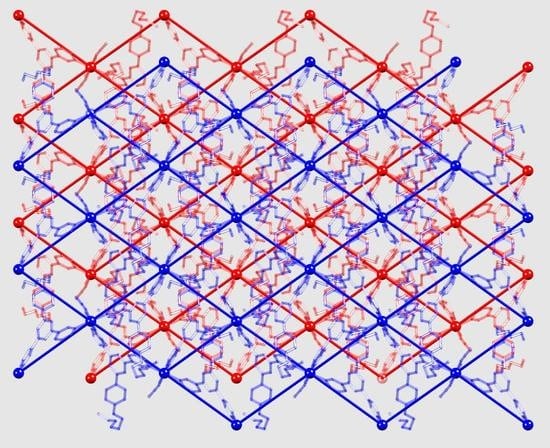
2.3. Crystal Structures
3. Materials and Methods
3.1. General
3.2. Compound 3
3.3. Crystal Growth of [{Co(2)2(NCS)2}.0.6CHCl3]n
3.4. Crystal Growth of [{Co(3)2(NCS)2}.4CHCl3.0.25H2O]n
3.5. Crystal Growth of [{Co(4)2(NCS)2}.4CHCl3]n
3.6. Crystal Growth of [Co2(5)4(NCS)4]n
3.7. Crystal Growth of [Co(6)2(NCS)2]n
3.8. Crystallography
3.9. [{Co(2)2(NCS)2}.0.6CHCl3]n
3.10. [{Co(3)2(NCS)2}.4CHCl3.0.25H2O]n
3.11. [{Co(4)2(NCS)2}.4CHCl3]n
3.12. [Co2(5)4(NCS)4]n
3.13. [Co(6)2(NCS)2]n
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Constable, E.C. 2,2′:6′,2″-Terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.; Winter, A.; Schlütter, F.; Schubert, U.S. Advances in the field of π-conjugated 2,2′:6′,2″-terpyridines. Chem. Soc. Rev. 2011, 40, 1459–1511. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; He, Y.; Shi, X.; Song, Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry. Coord. Chem. Rev. 2019, 385, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Agosti, A.; Kuna, E.; Bergamini, G. Divergent terpyridine-based coordination for the construction of photoactive supermolecular structures. Eur. J. Inorg. Chem. 2019, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Newkome, G.R. Terpyridine-based metallosupramolecular constructs: Tailored monomers to precise 2D-motifs and 3D-metallocages. Chem. Soc. Rev. 2018, 47, 3991–4016. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. More Hydra than Janus—Non-classical coordination modes in complexes of oligopyridine ligands. Coord. Chem. Rev. 2017, 350, 84–104. [Google Scholar] [CrossRef] [Green Version]
- Kröhnke, F. The specific synthesis of pyridines and oligopyridines. Synthesis 1976, 1–24. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A facile route to sterically hindered and non-hindered 4′-aryl-2,2′:6′,2″-terpyridines. Synlett 2005, 1251–1254. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The cambridge structural database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Housecroft, C.E. Divergent 4,2′:6′,4′′- and 3,2′:6′,3′′-Terpyridines as Linkers in 2- and 3-Dimensional Architectures. CrystEngComm 2015, 17, 7461–7468. [Google Scholar] [CrossRef] [Green Version]
- Rocco, D.; Manfroni, G.; Prescimone, A.; Klein, Y.M.; Gawryluk, D.J.; Constable, E.C.; Housecroft, C.E. Single and double-stranded 1D-coordination polymers with 4′-(4-alkyloxyphenyl)-3,2′:6′,3″-terpyridines and {Cu2(μ-OAc)4} or {Cu4(μ3-OH)2(μ-OAc)2(μ3-OAc)2(AcO-κO)2} motifs. Polymers 2020, 12, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Housecroft, C.E. 4,2′:6′,4″-Terpyridines: Diverging and diverse building blocks in coordination polymers and metallomacrocycles. Dalton Trans. 2014, 43, 6594–6604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Housecroft, C.E.; Constable, E.C. Ditopic and tetratopic 4,2′:6′,4″-Terpyridines as structural motifs in 2D- and 3D-coordination assemblies. Chimia 2019, 73, 462–467. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Liu, Y.; O’Keeffe, M. Regular Figures, Minimal Transitivity, and Reticular Chemistry. Isr. J. Chem. 2018, 58, 962–970. [Google Scholar] [CrossRef]
- Klein, Y.M.; Constable, E.C.; Housecroft, C.E.; Zampese, J.A.; Crochet, A. Greasy tails switch 1D-coordination [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)]npolymers to discrete [Zn2(OAc)4(4′-(4-ROC6H4)-4,2′:6′,4″-tpy)2] complexes. Cryst. Eng. Comm. 2014, 16, 9915–9929. [Google Scholar] [CrossRef] [Green Version]
- Constable, E.C.; Housecroft, C.E.; Vujovic, S.; Zampese, J.A. 2D → 2D Parallel interpenetration of (4,4) sheets constructed from a ditopic bis(4,2′:6′,4″-terpyridine). Cryst. Eng. Comm. 2014, 16, 3494–3497. [Google Scholar] [CrossRef] [Green Version]
- Vujovic, S.; Constable, E.C.; Housecroft, C.E.; Morris, C.D.; Neuburger, M.; Prescimone, A. Engineering 2D→2D parallel interpenetration using long alkoxy-chain substituents. Polyhedron 2015, 92, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Rocco, D.; Prescimone, A.; Klein, Y.M.; Gawryluk, D.J.; Constable, E.C.; Housecroft, C.E. Competition in coordination assemblies: 1D-coordination polymer or 2D-nets based on Co(NCS)2 and 4′-(4-methoxyphenyl)-3,2′:6′,3″-terpyridine. Polymers 2019, 11, 1224. [Google Scholar] [CrossRef] [Green Version]
- Rocco, D.; Housecroft, C.E.; Constable, E.C. Synthesis of terpyridines: Simple reactions—What could possibly go wrong? Molecules 2019, 24, 1799. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Zhang, Y.Z.; Yang, C.; Liu, E.; Golen, J.A.; Zhang, G. One-dimensional copper(II) coordination polymers built on 4′-substituted 4,2′:6′,4″- and 3,2′:6′,3″-terpyridines: Syntheses, structures and catalytic properties. Polyhedron 2016, 105, 115–122. [Google Scholar] [CrossRef]
- Cave, G.W.V.; Raston, C.L. Efficient synthesis of pyridines via a sequential solventless aldol condensation and Michael addition. J. Chem. Soc. Perkin Trans. 1 2001, 24, 3258–3264. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. 2-Dimensional networks assembled using 4′-functionalized 4,2′:6′,4″-terpyridines and Co(NCS)2. Polyhedron 2016, 103, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Klein, Y.M.; Prescimone, A.; Pitak, M.B.; Coles, S.J.; Constable, E.C.; Housecroft, C.E. Constructing chiral MOFs by functionalizing 4,2′:6′,4″-terpyridine with long-chain alkoxy domains: Rare examples of neb nets. Cryst. Eng. Comm. 2016, 18, 4704–4707. [Google Scholar] [CrossRef] [Green Version]
- SAINT. Software for the Integration of CCD Detector System Bruker Analytical X-ray System; Bruker axs:: Madison, WI, USA, 2013. [Google Scholar]
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with ShelXL. Acta Cryst. 2015, C27, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Chapuis, G. Superflip—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef] [Green Version]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A computer program for topological analysis of discrete electron densities. J. Appl. Cryst. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- LeBail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mat. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Cryst. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction patterns analysis. In Proceedings of the Seventh European Powder Diffraction Conference, Barcelona, Spain, 20–23 May 2000; pp. 118–123. [Google Scholar]













| Compound | Co–Ntpy/Å | Co–NNCS/Å | Ntpy–Co–Ntpy1/deg | NNCS–Co–NNCS/deg |
|---|---|---|---|---|
| [{Co(2)2(NCS)2}.0.6CHCl3]n | 2.172(5), 2.159(5) | 2.083(7) | 174.4(2) | 178.0(3) |
| [{Co(3)2(NCS)2}.4CHCl3.0.25H2O]n 2 | 2.211(6), 2.200(5), 2.183(6), 2.164(6) | 2.067(7), 2.084(7) | 175.5(2), 174.0(2) | 177.0(2) |
| [{Co(4)2(NCS)2}.4CHCl3]n 2 | 2.159(5), 2.172(5), 2.215(5), 2.229(5) | 2.075(6), 2.083(6) | 175.8(2), 173.78(19) | 177.5(2) |
| [Co2(5)4(NCS)4]n | 2.1693(18), 2.2175(18) | 2.0870(19) | 180.00(9), 180.0 | 180.0 |
| [Co(6)2(NCS)2]n 2 | 2.1857(19), 2.1789(19), 2.2237(18), 2.2356(18) | 2.0754(18), 2.0799(18) | 179.22(7), 178.59(7) | 179.24(7) |
| Compound | angle 11/deg | angle 21/deg |
|---|---|---|
| [{Co(2)2(NCS)2}.0.6CHCl3]n | 28.1 | 43.9 |
| [{Co(3)2(NCS)2}.4CHCl3.0.25H2O]n 2 | 22.6, 26.1 | 40.5, 40.6 |
| [{Co(4)2(NCS)2}.4CHCl3]n 2 | 28.5, 23.1 | 37.9, 38.1 |
| [Co2(5)4(NCS)4]n | 28.5 | 32.8 |
| [Co(6)2(NCS)2]n 2 | 29.7, 29.9 | 30.8, 33.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Directing 2D-Coordination Networks: Combined Effects of a Conformationally Flexible 3,2′:6′,3″-Terpyridine and Chain Length Variation in 4′-(4-n-Alkyloxyphenyl) Substituents. Molecules 2020, 25, 1663. https://doi.org/10.3390/molecules25071663
Rocco D, Prescimone A, Constable EC, Housecroft CE. Directing 2D-Coordination Networks: Combined Effects of a Conformationally Flexible 3,2′:6′,3″-Terpyridine and Chain Length Variation in 4′-(4-n-Alkyloxyphenyl) Substituents. Molecules. 2020; 25(7):1663. https://doi.org/10.3390/molecules25071663
Chicago/Turabian StyleRocco, Dalila, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2020. "Directing 2D-Coordination Networks: Combined Effects of a Conformationally Flexible 3,2′:6′,3″-Terpyridine and Chain Length Variation in 4′-(4-n-Alkyloxyphenyl) Substituents" Molecules 25, no. 7: 1663. https://doi.org/10.3390/molecules25071663