Abstract
Background
Asthma is a common chronic respiratory disease in children. In addition to medications, physical therapy is considered as a treatment strategy for asthma. We conducted this study to investigate the effects of physical therapy on lung function in children with asthma.
Methods
Three databases were searched. We conducted the meta-analysis for the forced expiratory volume in the first second in percent predicted values [FEV1(%pred)], the forced vital capacity in percent predicted values [FVC(%pred)], and the peak expiratory flow in percent predicted values [PEF(%pred)] by using a random effect model.
Results
Of the 6474 identified studies, 18 studies (16 in physical training, 2 in breathing exercise or inspiratory muscle training) were included in the systematic review and 11 studies (all in physical training) were included in the meta-analysis. The meta-analysis showed a significantly improved FVC(%pred) in the experimental group.
Conclusions
Physical training improved FVC(%pred) significantly in children with asthma. Further study is needed, especially on the effects of breathing exercise and inspiratory muscle training in children with asthma.
Impact
-
Our study reviewed the physical therapies for children with asthma and clarified whether and how these therapies affect them.
-
Our study found that physical training improved the forced vital capacity in percent predicted values [FVC(%pred)] significantly in asthmatic children.
-
Our study provided evidence that physical training could improve lung function in children with asthma, which is not identical to the Global Initiative for Asthma (GINA) guidelines.
Similar content being viewed by others
Introduction
Asthma is a common chronic respiratory disease seriously affecting patients’ life quality and quantity.1 As estimated by the Global Initiative for Asthma (GINA), there are 300 million people affected by asthma worldwide, leading to a great burden on the healthcare system and a huge economic loss.2 However, the etiology of asthma is not totally clear although some environmental risk factors, including allergen exposure, smoking exposure as well as air pollution exposure, and genetic risk factors, such as GATA binding protein 3 and Fc fragment of IgE receptor Ib, have been reported.3 Patients with asthma present symptoms with wheezing, cough, shortness of breath, and chest tightness resulting from airway hyperresponsiveness and airway remodeling.4 Acute exacerbation may pose a threat to the patient’s life, and they may need to be hospitalized as soon as possible.5
Asthma care is comprehensive, involving patient’s assessment, strategies’ adjustment, and treatments’ review.6 Pharmacologic therapy has been widely regarded as an important component of asthma treatment.7 Medications include control agents and relief agents, aiming to prevent or relieve asthma exacerbations, respectively.8 Besides, different types of physical therapy are considered as a part of asthma treatment due to the general health benefits.9 Physical training, breathing exercise, and inspiratory muscle training (IMT) are the three most relevant physical therapies for asthma.10
As reported, physical training is beneficial to physical and psychosocial health in healthy people.11 In asthmatic patients, it may improve their lung function by strengthening respiratory muscles, reducing airway inflammation, and increasing bronchioles’ patency.12 Breathing exercise can be performed in various forms such as the Papworth method and the Buteyko breathing technique,13 aiming to adapt asthmatic patients to an appropriate breathing pattern with a longer expiration and a lower respiratory rate, and thereby reduce hyperventilation and hyperinflation.14 There are three types of IMT, including normocapnic hyperpnea, flow-resistive loading, and pressure threshold loading.15 IMT is performed with a technique that can improve the strength and endurance of the diaphragm and the accessory inspiratory muscles.16
Although there are some published system review and meta-analysis evaluating the effects of physical therapy on patients with asthma, they did not exclude adults.10,17,18 Although some excluded adults, they involved only one type of physical therapy19,20 Thus we performed this systematic review and meta-analysis focusing on children only and involving at least one of the three most relevant physical therapies, namely, physical training, breathing exercise, and IMT. Our aims were to demonstrate the effects of physical therapy on lung function in children with asthma and provide evidence-based information for doctors on the choice of physical therapy for asthmatic children.
Methods
This system review and meta-analysis was registered at the international prospective register of systematic reviews, and the registration number is CRD42019121627. The study protocol has been published previously,21 and we reported this systematic review and meta-analysis in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement.22 No ethical approval was required for this study because there was no direct involvement of humans.
Search strategy
We searched three main databases from their inception to 30 November, 2018: PubMed, Embase, and the Cochrane Library. The search strategy in PubMed is described in Table 1. The same search strategy was used in Embase and the Cochrane Library based on different specific requirements. We also scanned the reference lists of studies and relevant systematic reviews for additional trials.
Selection criteria
We regarded studies as eligible for inclusion if they met the following criteria:
-
1.
Randomized controlled trials (RCTs) published in English.
-
2.
The study population included had to meet the age <18 years and the diagnosis as asthma by clearly defined or internationally recognized criteria.
-
3.
Apart from the treatment for the control group, at least one of the three most relevant physical therapies, which are physical training, breathing exercise, and IMT, should be applied to the experimental group with a minimum duration of 2 weeks.
-
4.
The study had to report lung function at the end of the intervention period: peak expiratory flow (PEF), forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC, and forced expiratory flow (FEF) in percent predicted values or absolute values.
Studies were excluded if it did not meet the inclusion criteria. Study selection was performed by two authors independently. We first reviewed the titles and abstracts of the papers, and the studies that were considered eligible were retrieved for full-text assessment. Any difference of opinion between the two authors was consulted with a third author.
Data extraction
Data were extracted from each selected study by two authors independently, including general information of the study (author, year, country), population information (number completed, age range (mean)), intervention (the type of physical therapy, duration), and outcomes reported (lung function). When extraction was finished, data were checked with each other by the two authors and the disputes were solved with the help of a third author.
Risk of bias assessment
The methodological quality of each included study was measured independently by two authors according to the Cochrane Collaboration’s tool.23 The following contents were evaluated: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.23 Each domain was judged to a high level, low level, or an unclear level.23 Any disagreements were solved by discussion or with the help of a third author.
Statistical analysis
Based on the selection criteria, the experimental group was treated with the therapies for the control group plus physical therapy in all included studies, meaning that the physical therapy was compared with no physical therapy. Studies reporting one of the following outcomes were included in the meta-analysis: the peak expiratory flow in percent predicted values [PEF(%pred)], the forced expiratory volume in the first second in percent predicted values [FEV1(%pred)], or the forced vital capacity in percent predicted values [FVC(%pred)]. Studies with a statistically significant difference at baseline between groups were excluded in the meta-analysis. We used the Revman Manager 5.3 to manage and analyze data. Mean ± standard deviation (SD) of post-intervention from groups were used to calculate the mean difference (MD) and the 95% confidence interval (CI) and weight between groups by using a random-effect model due to the large diversity of intervention. P < 0.05 was considered to be statistically significant. If studied reported pre mean ± SD and change mean ± SD, values were used to calculate post mean ± SD according to the Cochrane handbook.24 If studies reported the standard error of mean or the 95% CI, values were converted into SD. If two or more experimental groups were reported, values were combined. Subgroup analyses were not conducted because studies with breathing exercise or IMT did not satisfy the included criteria for meta-analysis.
We also assessed heterogeneity by χ2 test with P < 0.10 indicating statistical significance, and I2 test with I2 > 50% indicating moderate-to-high heterogeneity.25 Sensitivity analysis was performed by excluding one study each time sequentially and comparing the results by using a random-effect model and a fixed-effect model. We constructed a funnel plot and used Egger’s tests to assess publication bias in Stata 14.0, with P < 0.1 indicating significant bias. Quality of evidence was assessed based on the grading of recommendations assessment, development, and evaluation (GRADE) system.26
Results
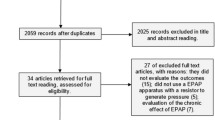
There were 6463 records identified through 3 databases by searching and 11 additional records by scanning the reference lists of studies and relevant systematic reviews. After duplicates were removed, there were 4656 records, of which 4571 records were excluded by screening the titles or/and abstracts. The full texts of the remaining 85 articles were assessed, of which 67 articles were excluded owing to inappropriate population, intervention, outcome, study design, or other reasons (on-going research, system review, publication language, etc.). Finally, 18 articles fulfilled the inclusion criteria, of which 11 articles reporting PEF(%pred), FEV1(%pred), or FVC(%pred) were included for meta-analysis. The results of study selection are summarized in a PRISMA (preferred reporting items for systematic reviews and meta-analyses) flow diagram (Fig. 1).
Study characteristics of the included studies
The characteristics of the included studies are summarized in Table 2. There were 18 studies involving 711 participants that completed the trials, of which 16 studies involving 631 participants performed physical training,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 1 study involving 30 participants performed breathing exercise,43 and 1 study involving 50 participants performed breathing exercise plus IMT.44 Studies were published between 1990 and 2018, of which 9 studies were between 2000 and 2009,28,30,31,34,37,38,40,41,44 and 6 studies were between 2010 and 2018,27,29,33,36,42,43 and the remaining 3 studies were before 2000.32,35,39 The studies were conducted in 12 countries, and most of them were in Europe and America. The age range (mean) of participants overall was 7–18 (11.71) years, in studies with breathing exercise or IMT it was 8–17 (12.59) years, and in studies with physical training it was 7–18 (10.83) years according to the existing data.
Risk of bias in the included studies
Risk of bias for each included study is summarized in Fig. 2, and each item presented as percentages across all included studies is presented in Fig. 3.
Allocation
All included studies were randomly allocated, but only five studies had described a well-randomized method and were judged as low risk of bias.27,29,34,41,43 Two studies reported a non-random component in the sequence generation process and were judged as high risk.35,44 Only one study supplied details regarding allocation concealment and was judged as low risk.27 Two studies were allocated based on alternation or a random number and were judged as high risk.35,41 The remaining studies were classified as unclear risk of bias for allocation concealment.
Blinding
The studies were all judged as high risk because it was impossible to avoid performance bias involving physical therapy. One study specified blinding of outcome assessment and was judged as low risk.27 The other studies were judged as an unclear risk of detection bias.
Incomplete outcome data
Six studies with a high rate of withdrawal were judged as high risk.27,31,33,34,41,42 Two studies specified withdrawals, but we judged them as low risk because missing outcome data were unlikely to be related to true outcomes.43,44 The other ten studies were judged as low risks.
Selective reporting
Although the study protocols of the included studies were not available, outcomes listed in the “Methods” section were all reported. Therefore, we judged them as low risk.
Other potential sources of bias
Three studies reported a statistically significant difference at baseline between groups and were judged as high risk.35,42,43 The remaining studies were judged as an unclear risk because there was no enough information provided.
Types of physical therapy
The summary of interventions is shown in Table 1. At least one of the three most relevant physical therapies was applied to the experimental group in 18 included studies. One study was conducted with breathing exercise for 12 weeks, with two 20-min sessions per week.43 One study was conducted with breathing exercise plus IMT for 7 consecutive weeks, with two 50-min sessions per week.44 The remaining 16 studies were conducted with physical training and variety modes were used, including walking, running, swimming, cycling, crawling, basketball, football, Tai Chi, and a combination of training.27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 The duration ranged from 5 weeks to 1 year except during school holidays. Most of the studies had 2 sessions or 3 sessions per week,27,28,30,31,33,34,35,36,37,38,39,40,41,42 except 2 studies that had 1 session per week.29,32 Each session lasted from 20 to 90 min, and most were 40–60 min per session.27,28,29,30,31,32,33,34,35,36,40,41,42
Outcome measures and findings
In each included study, the effects of physical therapy in the experimental group were compared with no physical therapy in the control group. Across the 18 studies included in the qualitative synthesis, six outcomes related to lung functions were assessed in percent predicted values (%pred) or absolute values, including FEV1,27,28,29,30,31,32,34,35,36,37,38,39,40,41,42,43,44 FVC,27,28,29,30,36,38,39,40,41,42 PEF,28,29,30,33,38,40,41,43,44 FEV1/FVC,28,29,31,40,42 FEF 50%,40 and FEF 25–75%.31,34,40,41,42 In this review, those reporting outcomes with FEV1(%pred), FVC(%pred), and PEF(%pred) were included in quantitative synthesis.
Forced expiratory volume in the first second
FEV1 was assessed in 16 studies, with 13 studies27,28,29,31,32,34,36,37,38,40,41,42,43 reporting in %pred and 7 studies30,35,37,38,39,41,42 reporting in absolute values. Six of those studies reported a significant improvement in FEV1.27,30,35,36,40,42
Meta-analysis
Of those studies reporting with %pred, 11 studies were included in the meta-analysis.27,28,29,31,32,34,36,37,38,40,41 Two studies were excluded because there was a significant difference in lung function at baseline between groups.42,43 The included studies were all conducted with physical training.
The corresponding forest plot is shown in Fig. 4. There was no statistically significant difference in FEV1(%pred) of post-intervention between two groups (MD, 1.77; 95% CI, −0.76 to 4.30; P = 0.17). The heterogeneity was acceptable (I2 = 32%).
Physical training versus control: a outcome: FEV1(%pred); b outcome: FVC(%pred); c outcome: PEF(%pred). FEV1(%pred) forced expiratory volume in the first second in percent predicted values, FVC(%pred) forced vital capacity in percent predicted values, PEF(%pred) peak expiratory flow in percent predicted values, SD standard deviation, CI confidence interval, IV inverse variance.
Forced vital capacity
FVC was the second most outcome measured in 10 studies, with 7 studies27,28,29,36,40,41,42 reporting in %pred and 5 studies30,38,39,41,42 reporting in absolute values. Five of those studies reported a significant improvement in FVC.27,29,30,36,42
Meta-analysis
Of those studies reporting with %pred, six studies were included in the meta-analysis.27,28,29,36,40,41 One study was excluded because there was a significant difference in lung function at baseline between two groups.42 The included studies were all conducted with physical training.
The corresponding forest plot is shown in Fig. 4. There was a statistically significant difference in FVC(%pred) of post-intervention between two groups (MD, 4.56; 95% CI, 1.33–7.79; P = 0.006). The heterogeneity was not detected (I2 = 0%).
Peak expiratory flow
PEF was evaluated in 9 studies, with 4 studies28,29,41,43 reporting in %pred and 6 studies30,33,38,40,41,44 reporting in absolute values. Four of those studies reported a significant improvement in PEF.30,33,40,44
Meta-analysis
Of those studies reporting with %pred, three studies were included in the meta-analysis.28,29,41 One study was excluded because there was a significant difference in lung function at baseline between two groups.43 The included studies were all conducted with physical training.
The corresponding forest plot is shown in Fig. 4. There was no statistically significant difference in PEF(%pred) of post-intervention between two groups (MD, 0.78; 95% CI, −5.24 to 6.97; P = 0.78). The heterogeneity was not detected (I2 = 0%).
Sensitivity analysis
We performed the sensitivity analysis by excluding one study each time sequentially and found that the pooled results were not changed significantly under any circumstances in a random-effect model or in a fixed-effect model, suggesting the stability of our meta-analysis. In addition, we found the study written by Abdelbasset in 201827 was the only source of heterogeneity in Fig. 4a.
Reporting bias
We constructed a funnel plot to evaluate the reporting bias and the shape presented symmetry basically (Fig. 5). Egger’s test was further conducted and the result indicated that there was no significant reporting bias (P = 0.797; Fig. 6, Table 3).
Quality of evidence
Quality of evidence based on the GRADE system is summarized in Table 4. Owing to the high risk of performance bias across all included studies, we downgraded one point on the risk of bias item. The result showed a moderate level of the evidence’s quality in all outcomes.
Discussion
The aim of this study was to investigate the effects of physical therapy on lung function in children with asthma. We included 18 studies in the systematic review, of which 16 were related to physical training and 2 were related to breathing exercise or IMT. In the meta-analysis, 11 of those related to physical training were included. No breathing exercise or IMT studies were included in the quantitative synthesis. Compared with the control groups, our results showed a significantly improved FVC(%pred) in groups with physical training (MD, 4.56; 95% CI, 1.33 to 7.79). No statistically significant difference was detected on FEV1(%pred) (MD, 1.77; 95% CI, −0.76 to 4.30) and PEF(%pred) (MD, 0.78; 95% CI, −5.24 to 6.97).
In the management of asthma, physical therapy is now considered as a treatment in addition to medications and is recommended in the GINA guideline.2 According to the guideline, physical training is encouraged for its general health benefits, and breathing exercise and IMT may be useful for asthma.2 However, whether and how these physical therapies benefit patients with asthma are not totally certain, especially in children. Besides, the results reported in different reviews are controversial. For example, one previous systematic review reported that physical training did not improve lung function in children with asthma by using FEV1(%pred) for meta-analysis,45 while another review found swimming improved FEV1(%pred).20
Our study involved the three most relevant physical therapies for asthma and conducted the meta-analysis by using FEV1(%pred), FVC(%pred), and PEF(%pred), which are the most commonly used parameters to evaluate lung function in clinical work. More importantly, the %pred could significantly reduce the heterogeneity among different trials and the pooled results became more comparable because lung function is related to participant’s characteristics such as region, race, age, and sex.
A key finding of this study is that physical training significantly improved FVC(%pred) in children with asthma. It provides statistical evidence to encourage children with asthma to take part in physical training. The possible reason why effects were found only for FVC(%pred) but not for FEV1(%pred) and PEF(%pred) is described below. The pathophysiology of asthma is complex and characterized by airway inflammation and bronchial hyperresponsiveness. It leads to not only the airway obstruction but also to the diminishing of the pulmonary elastic recoil. FVC(%pred) is a parameter that mainly reflects the pulmonary capacity, while FEV1(%pred) and PEF(%pred) are often used to evaluate the airway patency. Physical training can significantly improve pulmonary elastic recoil, resulting in an increase of pulmonary capacity. But the effects on reducing airway inflammation and bronchial hyperresponsiveness are probably not obvious. Thus the effects for FEV1(%pred) and PEF(%pred) were not found. In this study, we could not compare the effects of the three types of physical therapy in quantity and determine which one was more helpful due to the limited studies involving breathing exercise or IMT. Further RCTs involving breathing exercise or IMT are needed.
Eleven studies involving physical training were included in the meta-analysis. Even though the statistical heterogeneity was acceptable or not detected, methodological diversity still existed because it was not possible to blind the participants involving physical training. Besides, there was clinical diversity with respect to the intervention designs. More specifically, the training modes ranged from a single mode such as swimming or cycling to a complex mode including a variety of training, the duration range from 5 weeks to 1 year except during school holidays, the length of each session range from 20 to 60 min, and the frequency range from 1 to 3 sessions per week.
Furthermore, we found the study written by Abdelbasset in 201827 was the only source of heterogeneity in Fig. 4a during the sensitivity analysis. We reassessed this study and found that the pulmonary functions in both the experimental and control groups reported significant changes. It was likely that all participants were instructed to receive asthma medications regularly and home breathing exercise was recommended when they were receiving aerobic exercise training.
In summary, our study demonstrates the effects of physical training on lung function in children with asthma. But more research on the mode, the duration, and the frequency of physical training is needed. Despite the physical health, the effects of physical training on psychosocial health should not be neglected.
Limitations
There are some limitations to our study. First, all included studies have a high risk of performance bias because it was not possible to blind the participants involving physical training. Second, clinical heterogeneity exists due to the diversity of the intervention designs. Third, the outcome measures in this meta-analysis are too limited. It would be better if we could look at more outcome measures that could reflect the patient’s respiratory condition more, such as respiratory muscle strength and dyspnea.
Conclusion
This meta-analysis demonstrates that physical training significantly improved FVC(%pred) in children with asthma. This finding may support the therapy of physical training in asthmatic children. But further research involving the physical training mode, the duration, and the frequency is needed. Besides, more trials on the effects of breathing exercise and IMT in children with asthma should be conducted in the future.
References
Mims, J. W. Asthma: definitions and pathophysiology. Int. Forum Allergy Rhinol. 5(Suppl 1), S2–S6 (2015).
Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, Revised 2018. http://www.ginasthma.org (2018)
Toskala, E. & Kennedy, D. W. Asthma risk factors. Int. Forum Allergy Rhinol. 5(Suppl 1), S11–S16 (2015).
Papi, A., Brightling, C., Pedersen, S. E. & Reddel, H. K. Asthma. Lancet 391, 783–800 (2018).
Stenson, E. K., Tchou, M. J. & Wheeler, D. S. Management of acute asthma exacerbations. Curr. Opin. Pediatr. 29, 305–310 (2017).
Reddel, H. K. & Levy, M. L. The GINA asthma strategy report: what’s new for primary care? NPJ Prim. Care Respir. Med. 25, 15050 (2015).
Reddy, A. P. & Gupta, M. R. Management of asthma: the current US and European guidelines. Adv. Exp. Med. Biol. 795, 81–103 (2014).
National Institutes of Health. Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J. Allergy Clin. Immunol. 120, S94–S138 (2007).
Janssen, I. & Leblanc, A. G. Systematic review of the health benefits of physical activity and fitness in school-aged children and youth. Int. J. Behav. Nutr. Phys. Act. 7, 40 (2010).
Bruurs, M. L., van der Giessen, L. J. & Moed, H. The effectiveness of physiotherapy in patients with asthma: a systematic review of the literature. Respir. Med. 107, 483–494 (2013).
Weiss, M. R., Phillips, A. C. & Kipp, L. E. Effectiveness of a school-based fitness program on youths’ physical and psychosocial health outcomes. Pediatr. Exerc. Sci. 27, 546–557 (2015).
Eijkemans, M., Mommers, M., Draaisma, J. M., Thijs, C. & Prins, M. H. Physical activity and asthma: a systematic review and meta-analysis. PLoS ONE 7, e50775 (2012).
Ram, F. S., Holloway, E. A. & Jones, P. W. Breathing retraining for asthma. Respir. Med. 97, 501–507 (2003).
Bruton, A. & Thomas, M. The role of breathing training in asthma management. Curr. Opin. Allergy Clin. Immunol. 11, 53–57 (2011).
Shei, R. J., Paris, H. L., Wilhite, D. P., Chapman, R. F. & Mickleborough, T. D. The role of inspiratory muscle training in the management of asthma and exercise-induced bronchoconstriction. Phys. Sportsmed. 44, 327–334 (2016).
Illi, S. K., Held, U., Frank, I. & Spengler, C. M. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med. 42, 707–724 (2012).
Carson, K. V. et al. Physical training for asthma. Cochrane Database Syst. Rev. 9, Cd001116 (2013).
Silva, I. S. et al. Inspiratory muscle training for asthma. Cochrane Database Syst. Rev. 9, Cd003792 (2013).
Macedo, T. M., Freitas, D. A., Chaves, G. S., Holloway, E. A. & Mendonca, K. M. Breathing exercises for children with asthma. Cochrane Database Syst. Rev. 4, Cd011017 (2016).
Beggs, S. et al. Swimming training for asthma in children and adolescents aged 18 years and under. Cochrane Database Syst. Rev. 4, Cd009607 (2013).
Wang, Q., Zhang, W., Liu, L., Yang, W. & Liu, H. Effects of physical therapy on lung function in children with asthma: study protocol for a systematic review and meta-analysis. Medicine (Baltimore) 98, e15226 (2019).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 62, 1006–1012 (2009).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
Higgins, J. P. T. & Green, S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration. http://handbook.cochrane.org (2011).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002).
Balshem, H. et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 64, 401–406 (2011).
Abdelbasset, W. K., Alsubaie, S. F., Tantawy, S. A., Abo Elyazed, T. I. & Kamel, D. M. Evaluating pulmonary function, aerobic capacity, and pediatric quality of life following a 10-week aerobic exercise training in school-aged asthmatics: a randomized controlled trial. Patient Prefer. Adherence 12, 1015–1023 (2018).
Basaran, S. et al. Effects of physical exercise on quality of life, exercise capacity and pulmonary function in children with asthma. J. Rehabil. Med. 38, 130–135 (2006).
Carew, C. & Cox, D. W. Laps or lengths? The effects of different exercise programs on asthma control in children. Pediatr. Pulmonol. 55, 877–881 (2018).
Chang, Y. F., Yang, Y. H., Chen, C. C. & Chiang, B. L. Tai Chi Chuan training improves the pulmonary function of asthmatic children. J. Microbiol. Immunol. 41, 88–95 (2008).
Counil, F. P. et al. Training of aerobic and anaerobic fitness in children with asthma. J. Pediatr. 142, 179–184 (2003).
Edenbrandt, L., Ols’eni, L., Svenonius, E. & Jonson, B. Effect of physiotherapy in asthmatic children - a one year follow-up after physical training once a week. Acta Paediatr. Scand. 79, 973–975 (1990).
Latorre-Roman, P. A., Navarro-Martinez, A. V. & Garcia-Pinillos, F. The effectiveness of an indoor intermittent training program for improving lung function, physical capacity, body composition and quality of life in children with asthma. J. Asthma 51, 544–551 (2014).
Moreira, A. et al. Physical training does not increase allergic inflammation in asthmatic children. Eur. Respir. J. 32, 1570–1575 (2008).
Neder, J. A., Nery, L. E., Silva, A. C., Cabral, A. L. B. & Fernandes, A. L. G. Short term effects of aerobic training in the clinical management of moderate to severe asthma in children. Thorax 54, 202–206 (1999).
Onur, E. et al. The beneficial effects of physical exercise on antioxidant status in asthmatic children. Allergol. Immunopathol. (Madr.) 39, 90–95 (2011).
Silva, C. S. et al. Comparison of morning and afternoon exercise training for asthmatic children. Braz. J. Med. Biol. Res. 39, 71–78 (2006).
van Veldhoven, N. H. et al. Children with asthma and physical exercise: effects of an exercise programme. Clin. Rehabil. 15, 360–370 (2001).
Varray, A. L., Mercier, J. G., Terral, C. M. & Prefaut, C. G. Individualized aerobic and high intensity training for asthmatic children in an exercise readaptation program. Is training always helpful for better adaptation to exercise? Chest 99, 579–586 (1991).
Wang, J. S. & Hung, W. P. The effects of a swimming intervention for children with asthma. Respirology 14, 838–842 (2009).
Weisgerber, M. C., Guill, M., Weisgerber, J. M. & Butler, H. Benefits of swimming in asthma: effect of a session of swimming lessons on symptoms and PFTs with review of the literature. J. Asthma 40, 453–464 (2003).
Wicher, I. B. et al. Effects of swimming on spirometric parameters and bronchial hyperresponsiveness in children and adolescents with moderate persistent atopic asthma. J. Pediatr. (Rio J.) 86, 384–390 (2010).
Bignall, W. J. R., Luberto, C. M., Cornette, A. F., Haj-Hamed, M. & Cotton, S. Breathing retraining for African-American adolescents with asthma: a pilot study of a school-based randomized controlled trial. J. Asthma 52, 889–896 (2015).
Lima, E. V. et al. Inspiratory muscle training and respiratory exercises in children with asthma. J. Bras. Pneumol. 34, 552–558 (2008).
Joschtel, B. et al. Effects of exercise training on physical and psychosocial health in children with chronic respiratory disease: a systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 4, e000409 (2018).
Acknowledgements
This study is supported by the Sichuan Provincial Department of Science and Technology (No. 2013sz0040). The funders had no role in the design, execution, or writing of this protocol.
Author information
Authors and Affiliations
Contributions
Q.W. put forward the concept of this study. W.Z., W.Y., and L.L. were engaged in the study search, study selection, data extraction, risk of bias assessment, and data analysis. H.L. was responsible for project administration. W.Z. drafted the preliminary version of this manuscript. All authors critically reviewed, revised, and approved this final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, W., Wang, Q., Liu, L. et al. Effects of physical therapy on lung function in children with asthma: a systematic review and meta-analysis. Pediatr Res 89, 1343–1351 (2021). https://doi.org/10.1038/s41390-020-0874-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-0874-x
This article is cited by
-
An evidence gap map of interventions for noncommunicable diseases and risk factors among children and adolescents
Nature Medicine (2024)
-
Addition of respiratory exercises to conventional rehabilitation for children and adolescents with cerebral palsy: a systematic review and meta-analysis
World Journal of Pediatrics (2023)
-
The use of respiratory muscle training in patients with pulmonary dysfunction, internal diseases or central nervous system disorders: a systematic review with meta-analysis
Quality of Life Research (2023)
-
Compliance of functional exercises in school-age children with limb fractures: implication for nursing countermeasures
BMC Pediatrics (2022)