Abstract
Synovial sarcoma (SS) is an aggressive tumor that most often affects the deep soft tissues in young adults. Intrathoracic SS is rare and is associated with poor outcome, highlighting the urgent need for a novel therapeutic strategy. In the process of clinical sequencing, we identified two patients with intrathoracic SS harboring the BRAF V600E mutation. The patients were women aged 32 and 23 years, and both presented with SS18–SSX2-positive monophasic SS in the thoracic cavity. BRAF V600E mutations were detected by next generation sequencing, and validated immunohistochemically by diffuse intense positivity to BRAF V600E mutation-specific antibodies. The phosphorylated ERK (pERK) immunohistochemistry result was also positive. One patient received a combination therapy of dabrafenib and trametinib, which led to tumor shrinkage. However, the tumor growth progressed 7.5 months later with an additional NRAS Q61K mutation. Immunohistochemical screening of 67 archival SS tumor samples failed to identify additional samples with BRAF V600E mutation. However, 32% of BRAF V600E-negative cases was positive for pERK, and one of the six tumors showing the highest pERK expression harbored an FGFR2-activating mutation. This is the first report of targetable BRAF mutation in a small subset of SS. Our study suggests involvement of the mitogen-activated protein kinase pathway and the potential clinical implication of BRAF mutation screening in SS.
Similar content being viewed by others
Introduction
Synovial sarcoma (SS) is an aggressive tumor that most often affects young adults, and accounts for 5–10% of soft-tissue sarcomas. The majority of tumors arise in the deep soft tissues of extremities, with the remaining occurring in the head, neck, trunk wall, and internal trunk, including the thoracic cavity [1]. SS can be categorized as a more common monophasic spindle cell type and biphasic type with epithelial nests/glands. A poorly differentiated pattern may be present in a subset. It is genetically defined by SS18 gene fusions including SS18–SSX1, SS18–SSX2, and SS18–SSX4 [1]. The SS18–SSX protein exerts oncogenic activity through various mechanisms that disrupt epigenetic control. For example, the fusion protein binds to the SWI/SNF chromatin remodeling complex, resulting in the displacement of native SS18 and eviction of BAF47 (SMARCB1). It also interacts with KDM2B to bring together the SWI/SNF complex and PRC1.1 on the unmethylated CpG islands to aberrantly reactivate the expression of developmentally regulated genes that are otherwise repressed by PRC2 [2, 3]. The SMARCB1 eviction from the SWI/SNF complex can be visualized as an immunohistochemical reduction of SMARCB1 staining, which is a diagnostically helpful finding because it is specifically seen in 90% of SS samples [4, 5]. SS is otherwise genomically silent and additional genetic alterations are rare in the primary tumors, with uncommon secondary mutations including TP53, PTEN, CTNNB1, APC, SETD2, and FBXW7 [6].
Primary intrathoracic SS is uncommon, but SS might represent the most common sarcoma type in the thoracic cavity [7, 8]. The management of intrathoracic SS has numerous challenges, including late detection, large tumor size, high histological grade, high patient age, and difficulty to obtain adequate surgical margin [7, 9]. In addition, correct diagnosis can be delayed by a small sample size and a variety of histological mimics. Consequently, intrathoracic SS is associated with poorer outcome than its soft-tissue counterparts, with frequent recurrence and metastasis [7,8,9,10,11]. The prognosis of recurrent/metastatic SS remains poor [12, 13], highlighting the need for a novel therapeutic strategy.
Herein, we describe two patients with SS that harbored BRAF V600E mutation, for the first time to our knowledge. The tumors in both patients primarily involved the thoracic cavity and one of them responded well to the combined BRAF/MEK inhibition, until it ultimately recurred with an additional NRAS mutation.
Materials and methods
The study was approved by the Institutional Review Board of the National Cancer Center, Tokyo, Japan (2012–374, 2014–089).
Next generation sequencing
We performed targeted next generation sequencing (NGS) using the NCC Oncopanel to capture coding exons and the reported translocated introns of ~100 genes. The genes targeted by the assay are listed in Supplementary Table 1. This assay is currently used in clinical practice and a detailed protocol has been published previously [14, 15].
Immunohistochemistry
A paraffin section of 4-μm thickness was cut from the representative block of each tumor. Heat-induced epitope retrieval was performed using Targeted Retrieval Solution pH9 (Dako, Glostrup, Denmark). The endogenous peroxidase activity was blocked using 3% hydrogen peroxide. The primary antibodies used were BRAF V600E mutation-specific antibody (clone VE1, dilution 1:200; Spring Bioscience, Pleasanton, CA, USA) and phosphorylated ERK (pERK, #4370, dilution 1:400; Cell Signaling Technology, Danvers, MA, USA). The slides were incubated for 1 h at room temperature with the primary antibodies and subsequently labeled using the EnVision system (Dako). Mouse LINKER (Dako) was used for BRAF V600E staining. BRAF V600E staining was considered positive when diffuse cytoplasmic staining was observed. pERK staining was considered positive when nuclear staining was observed in ≥10% of cells.
Results
Case summary of BRAF-mutant SS
Case 1: A 32-year-old Japanese woman presented with chest pain resulting from a 12-cm mass in the right thoracic cavity (Fig. 1a). A thoracoscopic biopsy revealed a monophasic SS showing a classic spindle cell morphology (Fig. 1b). The immunohistochemical analysis showed focal-positive staining for epithelial membrane antigen and cytokeratin, whereas SMARCB1 expression was reduced. RNA sequencing revealed an SS18–SSX2 fusion transcript, which was validated by fluorescence in-situ hybridization using the SS18 break-apart probe (Fig. 1c). The patient received chemotherapy with doxorubicin and ifosfamide, which resulted in some tumor shrinkage. She underwent tumor resection and adjuvant chemotherapy with doxorubicin and ifosfamide, leading to a complete remission. After 18 months, a pulmonary metastasis appeared and the resected specimen showed a similar histology. The patient was enrolled in a phase I trial of trabectedin, which led to transient disease control. Following tumor progression, she was treated with ifosfamide monotherapy and pazopanib. Clinical NGS using NCC Oncopanel v2 on the resected pulmonary metastatic tumor revealed a BRAF V600E mutation (Fig. 1d), with a variant allele frequency of 24.8% (280/1129 reads) in case of a histologically estimated tumor cell ratio of 75%. The tumor showed diffuse intense immunohistochemical reactivity using BRAF V600E mutation-specific antibodies decorating virtually all tumor cells, both in the primary and metastatic sites (Fig. 1e). Immunohistochemistry for pERK was also positive in both primary and metastatic tumors (Fig. 1f). The patient was unable to receive BRAF inhibitors because such trials were not open at that time. She received supportive care and died of the disease 43 months after the initial presentation.
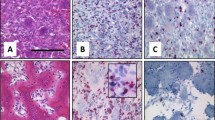
Computed tomography image showing a large mass in the right thoracic cavity (a, arrow). The tumor showed a classic histology of monophasic synovial sarcoma consisting of a fascicular growth of uniform spindle cells (b). RNA sequencing revealed an SS18–SSX2 fusion, which was confirmed by positive evidence of SS18 rearrangement using FISH (c, isolated red signals indicate SS18 rearrangement). Clinical next generation sequencing revealed a BRAF V600E mutation (d), which was supported by diffuse intense immunohistochemical reactivity using BRAF V600E mutation-specific antibody (e). Phosphorylated ERK immunohistochemistry was positive (f).
Case 2: A 23-year-old Japanese woman presented with Horner’s syndrome due to a 4.3-cm tumor in the superior mediastinum (Fig. 2a). The tumor was complicated by a hemothorax, which required immediate tumor resection. The tumor displayed classic monophasic SS histology (Fig. 2b), which was supported by RNA sequencing that revealed an SS18–SSX2 fusion transcript. This was further validated by reverse transcriptase polymerase chain reaction and Sanger sequencing (Fig. 2c). Five months later, she presented with pain in the right arm and shoulder due to local recurrence. She received a range of therapies including doxorubicin and ifosfamide, local irradiation, pazopanib, and ifosfamide monotherapy, all of which induced only transient tumor response with the subsequent regrowth. Clinical NGS using NCC Oncopanel v4 performed on the resected tumor revealed a BRAF V600E mutation (Fig. 2d), with a variant allele frequency of 53.8% (276/513 reads) in case of a histologically estimated tumor cell ratio of 100%. Immunohistochemically, the tumor showed diffuse intense positivity using BRAF V600E mutation-specific antibodies (Fig. 2e) decorating all tumor cells, and pERK staining was also positive (Fig. 2f).
Computed tomography image showing a mass in the superior thoracic cavity (a, arrow). The tumor showed classic histology of monophasic synovial sarcoma (b). RNA sequencing revealed an SS18–SSX2 fusion, which was validated by Sanger sequencing (c). Clinical next generation sequencing detected a BRAF V600E mutation (d), which was supported by diffuse intense immunohistochemical reactivity using BRAF V600E mutation-specific antibody (e). Phosphorylated ERK immunohistochemistry was positive (f).
BRAF-mutant SS responded to BRAF/MEK inhibition
Patient 2 received a combination therapy of dabrafenib (BRAF inhibitor, 150 mg BID) and trametinib (MEK inhibitor, trametinib 2 mg QD), which led to a partial response according to RECIST version 1.1 and the tumor was not detectable in 3 months (Fig. 3a, b). The tumor showed continuous remission until 7.5 months after BRAF/MEK inhibition, when it locally recurred (Fig. 3c). The biopsy of the recurrent tumor showed similar monophasic SS histology and was immunoreactive to BRAF V600E and pERK. NGS of this recurrent specimen detected the BRAF V600E mutation; in addition, it revealed an NRAS Q61K mutation, which was undetectable in the primary specimen.
Computed tomography image prior to the combination therapy showing a 2.1-cm recurrence in the right superior thoracic cavity (a, arrow). Following the administration of dabrafenib and trametinib, the mass shrank to 1.4 cm (−33% reduction, partial response) at 41 days. At 90 days, the mass was unmeasurable (b, arrow). The tumor recurred after 7.5 months (c, arrow). Biopsy of the recurrent tumor showed monophasic synovial sarcoma histology with BRAF V600E and a newly acquired NRAS Q61K as a mechanism of resistance.
BRAF V600E immunohistochemical screening of archival SS tissues
The recurrent identification of BRAF mutation in the two patients with SS prompted us to undertake a retrospective survey of archival SS tissues. We performed BRAF V600E immunohistochemical analysis of 67 SS tumor tissues originating from 67 patients. The diagnosis of all tumors was reviewed by a soft-tissue pathologist (AY) and confirmed by histological analyses in addition to the positive evidence of SS18 rearrangement by FISH and/or reduced immunoexpression of SMARCB1. The tested cases were enriched for thoracic primary tumors (N = 23) arising from the lung, pleura, mediastinum, or chest wall. No additional tumors positive for BRAF V600E immunoreactivity were found, and the estimated prevalence of BRAF V600E mutation in SS was up to 2.9% (2/69) in all anatomical sites and up to 8% (2/25) in the primary thoracic tumor.
pERK immunohistochemistry in the cohort lacking BRAF V600E
With a hypothesis that some SSs lacking BRAF V600E mutation might harbor other activating mechanisms of the mitogen-activated protein kinase (MAPK) pathway, we examined pERK immunohistochemistry in 53 available SS tumor tissues that lacked BRAF V600E immunoreactivity. We found that 17 cases showed positive staining with a range of 10–80%, whereas the remaining 68% showed negative (0% or <10%) staining. Of note, pERK-positive and -negative cohorts were not significantly different with regard to patient age, sex, tumor site (thoracic vs. non-thoracic), histological type (monophasic vs. biphasic), or overall survival. Among the 17 pERK-positive tumors, the amount and quality of six specimens were adequate for the NCC Oncopanel v4 assay. One of the six tumors showed an activating mutation of FGFR2 (c.C758G, p.P253R) with an allele frequency of 20.5% in case of a histologically estimated tumor cell rate of 60%, whereas the remaining five tumors harbored no mutations in the genes that were covered by the panel. The FGFR2-mutant tumor was a biphasic SS in the forearm of a 29-year-old woman.
Discussion
To the best of our knowledge, this is the first report to describe a BRAF V600E mutation in SS. BRAF mutation has not been detected in >180 SS tissues sequenced previously [2, 16,17,18,19,20,21,22,23]. BRAF is a member of the RAF family of serine/threonine kinases that plays key roles in the canonical MAPK cascade, which conveys signals from the surface receptor tyrosine kinase through RAS toward the downstream MEK and ERK. The oncogenic BRAF mutation is reported in a wide variety of human neoplasms, of which BRAF V600E is the most common type accounting for >90% of all reported mutations. The estimated frequency of BRAF V600E mutation in SS is similar to other solid tumors in which small subsets of cases harbor this alteration, according to historical and recent observations [24,25,26]. From a diagnostic standpoint, the BRAF V600E mutation in spindle cell “sarcoma” is often suspected as a clue for misdiagnosed sarcomatoid (“dedifferentiated”) malignant melanoma [27, 28]. Although this suspicion is reasonable for many cases, our study shows that the mutation can also rarely occur in bona fide sarcomas. Of note, SW982 “synovial sarcoma” cell line with BRAF V600E mutation [29] likely did not originate from SS because it lacks SS18–SSX fusion [30].
Rare (<5%) but recurrent presence of BRAF V600E mutations in SS might open a potential avenue to targeted therapy. Diffuse intense immunoreactivity using BRAF V600E-specific antibodies, observed in all tested samples (i.e., primary, recurrence, and/or metastasis) of both patients, suggests an early driver role of BRAF mutation, rather than being acquired later in a small subclone, although the mutation is likely a secondary event to initiating oncogenic SS18–SSX fusion. Combined therapy using dabrafenib and trametinib has been proven effective for advanced malignant melanoma, anaplastic thyroid carcinoma, and non-small cell lung carcinoma carrying BRAF mutations [31,32,33,34], and is expected to be promising in other tumor types [35]. Patient 2 indeed showed response to combined BRAF/MEK inhibition; however, the tumor ultimately recurred 7.5 months later, with an additional activating NRAS mutation. This pattern of initial response and subsequent progression to targeted therapy is similar to observations in other tumor types. NRAS mutation is a known cause of resistance to BRAF/MEK inhibition in melanoma and non-small cell lung carcinoma with BRAF mutation [36, 37], and this particular NRAS Q61K has been reported in 2–8% of BRAF-mutant melanoma cases resistant to single-agent BRAF inhibition [38,39,40,41] or combined BRAF/MEK inhibition [37]. Although it is presently unclear how NRAS mutation confers resistance to BRAF/MEK inhibition [36], the proposed hypotheses based on preclinical NRAS-mutant melanoma models include compensatory signaling via other RAF family members [42] and/or activation of the PI3K pathway [43, 44].
In the present report, both BRAF-mutant SSs displayed a considerable degree of clinicopathological similarity, including monophasic subtype, SS18–SSX2 fusion, primary intrathoracic location, and patient age and sex with both of them being young adult women. Whether these shared features indicate any significance remains to be determined, but focusing on these particular clinicopathological aspects might be worthwhile to identify additional cases belonging to this molecular class.
Both BRAF-mutant SSs displayed high pERK immunoexpression. Because ERK phosphorylation is widely considered as a marker of MAPK pathway activation, this finding is consistent with the aberrantly enhanced signal transduction via this pathway induced by BRAF mutation. The finding is similar to some previous studies, in which pERK immunohistochemistry was uniformly positive in tumors with known genetic abnormalities that activate the MAPK pathway, such as those involving BRAF and MEK1 [45, 46]. We thus hypothesized that SSs lacking BRAF V600E mutation yet overexpressing pERK might harbor mutations in genes that encode other members of the MAPK pathway. We first showed by immunohistochemistry that pERK is variably expressed in a third of clinical tumor samples of SS, and, in one of the six such cases tested, we identified an activating FGFR2 mutation as a potential cause of ERK activation. Although FGFR2 mutation has not been reported in SS [2, 16, 17, 21, 22], FGFR2 P253R is a known oncogenic mutation that has been recurrently detected in human tumors including endometrial and lung carcinomas, and it is likely a gain-of-function mutation that alters extracellular domain and enhances the affinity to ligands [47, 48].
Our study is limited by a small number of patients with BRAF-mutant SS. Although one patient responded to targeted therapy, whether the response is specific for this small molecular subset remains unknown. In addition, subsequent BRAF screening was performed primarily by immunohistochemistry owing to a limited number of materials available. Hence, cases with alternative BRAF mutations might have been missed, although the lack thereof was confirmed by NGS in six cases with the highest pERK expression. Finally, it is unclear whether screening by pERK staining is effective to identify cases with actionable gene abnormalities within the MAPK pathway, because the correlation between gene mutation and pERK immunoexpression has not been consistent in the literature, and there are reports of BRAF-mutant colorectal carcinomas or melanomas that were negative for pERK immunohistochemistry [49, 50]. In addition, we noted that pERK staining tended to be more enhanced at the periphery than the center of the tissue section, as seen in a previous report [45], suggesting a contribution of pre-analytic parameters such as fixation time and time to fixation. Further studies are required to estimate the actual prevalence of BRAF-mutant SS and to accurately measure the therapeutic effect of targeted therapy.
In conclusion, we discovered that BRAF V600E mutation is present in a small subset of intrathoracic SS, and reported that one of such tumors showed transient response to BRAF/MEK inhibition. Our study suggests a role of MAPK pathway and potential clinical implication of BRAF mutation screening in SS.
References
Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F. WHO Classification of tumours of Soft Tissue and Bone. Lyon: IARC; 2013.
McBride MJ, Pulice JL, Beird HC, Ingram DR, D’Avino AR, Shern JF, et al. The SS18-SSX fusion oncoprotein hijacks BAF complex targeting and function to drive synovial sarcoma. Cancer Cell. 2018;33:1128–41. e1127.
Banito A, Li X, Laporte AN, Roe JS, Sanchez-Vega F, Huang CH, et al. The SS18-SSX oncoprotein hijacks KDM2B-PRC1.1 to drive synovial sarcoma. Cancer Cell. 2018;33:527–41. e528.
Ito J, Asano N, Kawai A, Yoshida A. The diagnostic utility of reduced immunohistochemical expression of SMARCB1 in synovial sarcomas: a validation study. Hum Pathol. 2016;47:32–37.
Arnold MA, Arnold CA, Li G, Chae U, El-Etriby R, Lee CC, et al. A unique pattern of INI1 immunohistochemistry distinguishes synovial sarcoma from its histologic mimics. Hum Pathol. 2013;44:881–7.
Nielsen TO, Poulin NM, Ladanyi M. Synovial sarcoma: recent discoveries as a roadmap to new avenues for therapy. Cancer Discov. 2015;5:124–34.
Begueret H, Galateau-Salle F, Guillou L, Chetalille B, Brambilla E, Vignaoud JM, et al. Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol. 2005;29:339–46.
Lan T, Chen H, Xiong B, Zhou T, Peng R, Chen M, et al. Primary pleuropulmonary and mediastinal synovial sarcoma: a clinicopathologic and molecular study of 26 genetically confirmed cases in the largest institution of southwest China. Diagn Pathol. 2016;11:62.
Essary LR, Vargas SO, Fletcher CD. Primary pleuropulmonary synovial sarcoma: reappraisal of a recently described anatomic subset. Cancer. 2002;94:459–69.
Hartel PH, Fanburg-Smith JC, Frazier AA, Galvin JR, Lichy JH, Shilo K, et al. Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series. Mod Pathol. 2007;20:760–9.
Kim GH, Kim MY, Koo HJ, Song JS, Choi C. Primary pulmonary synovial sarcoma in a tertiary referral center: clinical characteristics, CT, and 18F-FDG PET findings, with pathologic correlations. Medicine. 2015;94:e1392.
Palmerini E, Staals EL, Alberghini M, Zanella L, Ferrari C, Benassi MS, et al. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer. 2009;115:2988–98.
Takenaka S, Ueda T, Naka N, Araki N, Hashimoto N, Myoui A, et al. Prognostic implication of SYT-SSX fusion type in synovial sarcoma: a multi-institutional retrospective analysis in Japan. Oncol Rep. 2008;19:467–76.
Sunami K, Ichikawa H, Kubo T, Kato M, Fujiwara Y, Shimomura A, et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: a hospital-based study. Cancer Sci. 2019;110:1480–90.
Tanabe Y, Ichikawa H, Kohno T, Yoshida H, Kubo T, Kato M, et al. Comprehensive screening of target molecules by next-generation sequencing in patients with malignant solid tumors: guiding entry into phase I clinical trials. Mol Cancer. 2016;15:73.
Barretina J, Taylor BS, Banerji S, Ramos AH, Lagos-Quintana M, DeCarolis P, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–21.
Joseph CG, Hwang H, Jiao Y, Wood LD, Kinde I, Wu J, et al. Exomic analysis of myxoid liposarcomas, synovial sarcomas, and osteosarcomas. Genes Chromosomes Cancer. 2014;53:15–24.
Teng HW, Wang HW, Chen WM, Chao TC, Hsieh YY, Hsih CH, et al. Prevalence and prognostic influence of genomic changes of EGFR pathway markers in synovial sarcoma. J Surg Oncol. 2011;103:773–81.
Je EM, An CH, Yoo NJ, Lee SH. Mutational analysis of PIK3CA, JAK2, BRAF, FOXL2, IDH1, AKT1 and EZH2 oncogenes in sarcomas. APMIS. 2012;120:635–9.
Qi Y, Wang N, Pang LJ, Zou H, Hu JM, Zhao J, et al. Identification of potential mutations and genomic alterations in the epithelial and spindle cell components of biphasic synovial sarcomas using a human exome SNP chip. BMC Med Genomics. 2015;8:69.
Vlenterie M, Hillebrandt-Roeffen MH, Flucke UE, Groenen PJ, Tops BB, Kamping EJ, et al. Next generation sequencing in synovial sarcoma reveals novel gene mutations. Oncotarget. 2015;6:34680–90.
The Cancer Genome Atlas Research Network. Electronic address edsc, cancer genome atlas research N. Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171:950–65. e928.
Xing Z, Wei L, Jiang X, Conroy J, Glenn S, Bshara W, et al. Analysis of mutations in primary and metastatic synovial sarcoma. Oncotarget. 2018;9:36878–88.
Allen A, Qin ACR, Raj N, Wang J, Uddin S, Yao Z, et al. Rare BRAF mutations in pancreatic neuroendocrine tumors may predict response to RAF and MEK inhibition. PLoS ONE. 2019;14:e0217399.
Behling F, Barrantes-Freer A, Skardelly M, Nieser M, Christians A, Stockammer F, et al. Frequency of BRAF V600E mutations in 969 central nervous system neoplasms. Diagn Pathol. 2016;11:55.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
Agaimy A, Specht K, Stoehr R, Lorey T, Makl B, Niedobitek G, et al. Metastatic malignant melanoma with complete loss of differentiation markers (undifferentiated/dedifferentiated melanoma): analysis of 14 patients emphasizing phenotypic plasticity and the value of molecular testing as surrogate diagnostic marker. Am J Surg Pathol. 2016;40:181–91.
Cipriani NA, Letovanec I, Hornicek FJ, Mullen JT, Duan Z, Borger DR, et al. BRAF mutation in ‘sarcomas’: a possible method to detect de-differentiated melanomas. Histopathology. 2014;64:639–46.
Becerikli M, Jacobsen F, Rittig A, Kohne W, Nambiar S, Mirmohammadsadegh A, et al. Growth rate of late passage sarcoma cells is independent of epigenetic events but dependent on the amount of chromosomal aberrations. Exp Cell Res. 2013;319:1724–31.
Teicher BA, Polley E, Kunkel M, Evans D, Silvers T, Delosh R, et al. Sarcoma cell line screen of oncology drugs and investigational agents identifies patterns associated with gene and microRNA expression. Mol Cancer Ther. 2015;14:2452–62.
Planchard D, Besse B, Groen HJM, Souquet PJ, Quiox E, Baik CS, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016;17:984–93.
Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland A, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol. 2017;18:1307–16.
Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl J Med. 2015;372:30–9.
Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J Clin Oncol. 2018;36:7–13.
Salama AKS, Li S, Macrae ER, Park J, Mitchell EP, Zwiebel JA, et al. Dabrafenib and trametinib in patients with tumors with BRAF V600E/K mutations: results from the molecular analysis for therapy choice (MATCH) Arm H. J Clin Oncol. 2019;37:3002.
Abravanel DL, Nishino M, Sholl LM, Ambrogio C, Awad MM. An acquired NRAS Q61K mutation in BRAF V600E-mutant lung adenocarcinoma resistant to dabrafenib plus trametinib. J Thorac Oncol. 2018;13:e131–3.
Long GV, Fung C, Menzies AM, Pupo GM, Carlino MS, Hyman J, et al. Increased MAPK reactivation in early resistance to dabrafenib/trametinib combination therapy of BRAF-mutant metastatic melanoma. Nat Commun. 2014;5:5694.
Johnson DB, Menzies AM, Zimmer L, Eroglu Z, F YE, Zhao S, et al. Acquired BRAF inhibitor resistance: A multicenter meta-analysis of the spectrum and frequencies, clinical behaviour, and phenotypic associations of resistance mechanisms. Eur J Cancer. 2015;51:2792–9.
Nazarian R, Shi H, Wang Q, Xiangju K, Koya RC, Lee H, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature. 2010;468:973–7.
Rizos H, Menzies AM, Pupo GM, Carlino MS, Fung C, Hyman J, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res. 2014;20:1965–77.
Van Allen EM, Wagle N, Sucker A, Treacy DJ, Johannesssen CM, Goetz EM, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Disco. 2014;4:94–109.
Dorard C, Estrada C, Barbotin C, Larcher M, Garancher A, Leloup J, et al. RAF proteins exert both specific and compensatory functions during tumour progression of NRAS-driven melanoma. Nat Commun. 2017;8:15262.
Atefi M, von Euw E, Attar N, Ng C, Chu C, Guo D, et al. Reversing melanoma cross-resistance to BRAF and MEK inhibitors by co-targeting the AKT/mTOR pathway. PLoS ONE. 2011;6:e28973.
Petit V, Raymond J, Alberti C, Pouteaux M, Gallagher SJ, Nguyen MQ, et al. C57BL/6 congenic mouse NRAS(Q61K) melanoma cell lines are highly sensitive to the combination of Mek and Akt inhibitors in vitro and in vivo. Pigment Cell Melanoma Res. 2019;32:829–41.
Kao YC, Ranucci V, Zhang L, Sung Y, Athanasian EA, Swanson D, et al. Recurrent BRAF gene rearrangements in myxoinflammatory fibroblastic sarcomas, but not hemosiderotic fibrolipomatous tumors. Am J Surg Pathol. 2017;41:1456–65.
Shanmugam V, Craig JW, Hornick JL, Morgan EA, Pinkus GS, Pozdnyakova O. Cyclin D1 is expressed in neoplastic cells of langerhans cell histiocytosis but not reactive Langerhans cell proliferations. Am J Surg Pathol. 2017;41:1390–6.
Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR landscape in cancer: analysis of 4,853 tumors by next-generation sequencing. Clin Cancer Res. 2016;22:259–67.
Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C, et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci USA. 2008;105:8713–7.
Houben R, Vetter-Kauczok CS, Ortmann S, Rapp UR, Broecker EB, Becker JC. Phospho-ERK staining is a poor indicator of the mutational status of BRAF and NRAS in human melanoma. J Investig Dermatol. 2008;128:2003–12.
Holck S, Bonde J, Pedersen H, Petersen AA, Chaube A, Nielsen HJ, et al. Localization of active, dually phosphorylated extracellular signal-regulated kinase 1 and 2 in colorectal cancer with or without activating BRAF and KRAS mutations. Hum Pathol. 2016;54:37–46.
Acknowledgements
The authors thank Mses. Sachiko Miura, Toshiko Sakaguchi, Chizu Kina, Sachiyo Mitani, and Erika Arakawa for superb technical assistance.
Funding
This work was supported in part by National Cancer Center Research and Development Fund (29-A-2, 30-A-6), Japan Agency for Medical Research and Development (JP18kk0205004, JP19ck0106257, JP19lk1403003), and JSPS Grant-in-Aid for Young Scientists (18K15108).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
TKo is a recipient of a collaborative research grant from the Sysmex Corporation. NY is a recipient of research grants from the Astellas, Chugai, Eisai, Taiho, BMS, Pfizer, Novartis, Eli Lilly, AbbVie, Daiichi-Sankyo, Bayer, Boehringer Ingelheim, Kyowa-Hakko Kirin, Takeda, ONO, Janssen Pharma, MSD, and MERCK. NY also serves as an advisor for Eisai, Takeda, Otsuka, Boehringer Ingelheim, Cimic, and Chugai, and as a speaker for BMS, Pfizer, AstraZeneca, Eli Lilly, ONO, Chugai, and Sysmex. All remaining authors have declared no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Watanabe, S., Shimomura, A., Kubo, T. et al. BRAF V600E mutation is a potential therapeutic target for a small subset of synovial sarcoma. Mod Pathol 33, 1660–1668 (2020). https://doi.org/10.1038/s41379-020-0530-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0530-3
This article is cited by
-
Upregulation of ERK-EGR1-heparanase axis by HDAC inhibitors provides targets for rational therapeutic intervention in synovial sarcoma
Journal of Experimental & Clinical Cancer Research (2021)
-
Correlating SS18-SSX immunohistochemistry (IHC) with SS18 fluorescent in situ hybridization (FISH) in synovial sarcomas: a study of 36 cases
Virchows Archiv (2021)