Estimating Plant Nitrogen Concentration of Maize Using a Leaf Fluorescence Sensor across Growth Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Soil Description
2.2. Experimental Design
2.3. Dualex 4 Sensor Data Collection, Plant Sampling, and Measurements
2.4. Statistical Analysis
3. Results
3.1. Interrelationships of SLNC, TLNC, and PNC
3.2. Effects of Soil Type, Growth Stage, and N Rate on Maize TLNC and PNC
3.3. Effects of Soil Type, Growth Stage, and N Rate on Dualex 4 Parameters
3.4. Relationships between Dualex 4 Parameters and TLNC or PNC
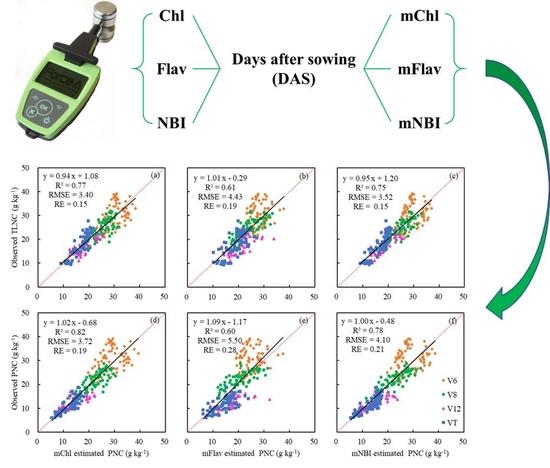
3.5. The Estimation of PNC Using Different Modified Dualex 4 Parameters
4. Discussion
4.1. Feasibility of Estimating Maize N Status Using Single Leaf-based Dualex 4 Parameters
4.2. Main Factor(s) Affecting the Establishment of the General Model and the Best Parameter(s) for PNC Estimation
4.3. The Most Suitable Leaf Position for Sensing Measurements and PNC Estimation
4.4. Implications for Practical Application and Future Research Needs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Cultured Aquatic Species Information Programme, Epinephelus Coioides. Available online: http://www.fao.org (accessed on 28 June 2019).
- Gu, B.; Ju, X.; Wu, Y.; Erisman, J.W.; Bleeker, A.; Reis, S.; Smith, R.I. Cleaning up nitrogen pollution may reduce future carbon sinks. Glob. Environ. Chang. 2018, 48, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Chen, X.; Vitousek, P. An experiment for the world. Nature 2013, 497, 33–35. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Archontoulis, S.V.; Lobell, D.B. How much will precision nitrogen management pay off? An evaluation based on simulating thousands of corn fields over the US corn-belt. Field Crop. Res. 2019, 240, 12–22. [Google Scholar] [CrossRef]
- Wang, X.; Miao, Y.; Dong, R.; Chen, Z.; Guan, Y.; Yue, X.; Fang, Z.; Mulla, D.J. Developing active canopy sensor-based precision nitrogen management strategies for corn in Northeast China. Sustainability 2019, 11, 706. [Google Scholar]
- Kyveryga, P.M.; Blackmer, A.M.; Zhang, J. Characterizing and classifying variability in corn yield response to nitrogen fertilization on subfield and field scales. Agron. J. 2009, 101, 269–277. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.F.; Guevara-Gonzalez, R.G.; Contreras-Medina, L.M.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef] [PubMed]
- Corti, M.; Cavalli, D.; Cabassi, G.; Gallina, P.M.; Bechini, L. Does remote and proximal optical sensing successfully estimate maize variables? A review. Eur. J. Agron. 2018, 99, 37–50. [Google Scholar] [CrossRef]
- Pinter, P.J.; Hatfield, J.L.; Schepers, J.S.; Barnes, E.M.; Moran, M.S.; Daughtry, C.S.T.; Upchurch, D.R. Remote sensing for crop management. Photogramm. Eng. Remote. Sens. 2003, 69, 647–664. [Google Scholar] [CrossRef] [Green Version]
- Mulla, D.J. Twenty-five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Nigon, T.J.; Mulla, D.J.; Rosen, C.J.; Cohen, Y.; Alchanatis, V.; Rud, R. Evaluation of the nitrogen sufficiency index for use with high resolution, broadband aerial imagery in a commercial potato field. Precis. Agric. 2014, 15, 202–226. [Google Scholar] [CrossRef]
- Delloye, C.; Weiss, M.; Defourny, P. Retrieval of the canopy chlorophyll content from Sentinel-2 spectral bands to estimate nitrogen uptake in intensive winter wheat cropping systems. Remote. Sens. Environ. 2018, 216, 245–261. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef] [Green Version]
- Kovács, P.; Vyn, T.J. Relationships between ear-leaf nutrient concentrations at silking and corn biomass and grain yields at maturity. Agron. J. 2017, 109, 2898–2906. [Google Scholar] [CrossRef] [Green Version]
- Gaju, O.; Allard, V.; Martre, P.; Le Gouis, J.; Moreau, D.; Bogard, M.; Hubbart, S.; Foulkes, M.J. Nitrogen partitioning and remobilization in relation to leaf senescence, grain yield and grain nitrogen concentration in wheat cultivars. Field Crop Res. 2014, 155, 213–223. [Google Scholar] [CrossRef]
- Isfan, D.; Zizka, J.; D’Avignon, A.; Deschênes, M. Relationships between nitrogen rate, plant nitrogen concentration, yield and residual soil nitrate-nitrogen in silage corn. Commun. Soil Sci. Plant Anal. 1995, 26, 2531–2557. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Moigne, M.L. Nondestructive diagnostic test for nitrogen nutrition of grapevine (Vitis vinifera L.) based on Dualex leaf-clip measurements in the field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Zhao, C.; Li, Z.; Yang, G.; Yang, W. Estimating total leaf nitrogen concentration in winter wheat by canopy hyperspectral data and nitrogen vertical distribution. J. Integr. Agric. 2019, 18, 1562–1570. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, B.; Tan, C.; Yu, W.; Gnyp, M.L.; Lenz-Wiedemann, V.I.S.; Rascher, U.; et al. Satellite remote sensing-based in-season diagnosis of rice nitrogen status in Northeast China. Remote Sens. 2015, 7, 10646–10667. [Google Scholar] [CrossRef] [Green Version]
- Longchamps, L.; Khosla, R. Early detection of nitrogen variability in corn using fluorescence. Agron. J. 2014, 106, 511–518. [Google Scholar] [CrossRef]
- Heege, H.J.; Reusch, S.; Thiessen, E. Prospects and results for optical systems for site-specific on-the-go control of nitrogen-top-dressing in Germany. Precis. Agric. 2008, 9, 115–131. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Cerovic, Z.G.; Goulas, Y.; Montpied, P.; Demotes-Mainard, S.; Bidel, L.P.; Moya, I.; Dreyer, E. Relationships between optically assessed polyphenols and chlorophyll contents, and leaf mass per area ratio in woody plants: A signature of the carbon-nitrogen balance within leaves? Plant Cell Environ. 2006, 29, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, Z.G.; Ounis, A.; Cartelat, A.; Latouche, G.; Khosla, R. The use of chlorophyll fluorescence excitation spectra for the non-destructive in situ assessment of UV-absorbing compounds in leaves. Plant Cell Environ. 2002, 25, 1663–1676. [Google Scholar] [CrossRef]
- Barnes, P.W.; Searles, P.S.; Ballaré, C.L.; Ryel, R.J.; Caldwell, M.M. Non-invasive measurements of leaf epidermal transmittance of UV radiation using chlorophyll fluorescence: Field and laboratory studies. Physiol. Plant. 2000, 109, 274–283. [Google Scholar] [CrossRef]
- Dong, T.; Shang, J.; Chen, J.; Liu, J.; Qian, B.; Ma, B.; Morrison, M.J.; Zhang, C.; Liu, Y.; Shi, Y.; et al. Assessment of portable chlorophyll meters for measuring crop leaf chlorophyll concentration. Remote Sens. 2019, 11, 2706. [Google Scholar] [CrossRef] [Green Version]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L.; Barbottin, A.; Jeuffroy, M.H.; Gate, P.; Agati, G.; et al. Optically assessed contents of leaf polyphenolics and chlorophyll as indicators of nitrogen deficiency in wheat (Triticum aestivum L.). Field Crop Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Evaluation of optical sensor measurements of canopy reflectance and of leaf flavonols and chlorophyll contents to assess crop nitrogen status of muskmelon. Eur. J. Agron. 2014, 58, 39–52. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Bélec, C. Evaluation of the Dualex for the assessment of corn nitrogen status. J. Plant Nutr. 2007, 30, 1355–1369. [Google Scholar] [CrossRef]
- Goulas, Y.; Cerovic, Z.G.; Cartelat, A.; Moya, I. Dualex: A new instrument for field measurements of epidermal ultraviolet absorbance by chlorophyll fluorescence. Appl. Opt. 2004, 43, 4488–4496. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Bélec, C. Performance of Dualex in spring wheat for crop nitrogen status assessment, yield prediction and estimation of soil nitrate content. J. Plant Nutr. 2009, 33, 57–70. [Google Scholar] [CrossRef]
- Apostol, S.; Viau, A.A.; Tremblay, N.; Briantais, J.-M.; Prasher, S.; Parent, L.-E.; Moya, I. Laser-induced fluorescence signatures as a tool for remote monitoring of water and nitrogen stresses in plants. Can. J. Remote Sens. 2003, 29, 57–65. [Google Scholar] [CrossRef]
- Xu, J.; Cai, H.; Wang, X.; Ma, C.; Lu, Y.; Ding, Y.; Wang, X.; Chen, H.; Wang, Y.; Saddique, Q. Exploring optimal irrigation and nitrogen fertilization in a winter wheat-summer maize rotation system for improving crop yield and reducing water and nitrogen leaching. Agric. Water. Manage. 2020, 228, 105904. [Google Scholar] [CrossRef]
- Carolina, S.P.; Crossa, J.L.; Bonnett, D.; Yamaguchi-Shinozaki, K.; Reynolds, M.P. Phenotyping transgenic wheat for drought resistance. J. Exp. Bot. 2012, 63, 1799–1808. [Google Scholar] [CrossRef] [Green Version]
- Scharf, P.C.; Kitchen, N.R.; Sudduth, K.A.; Davis, J.G. Spatially variable corn yield is a weak predictor of optimal nitrogen rate. Soil Sci. Soc. Am. J. 2006, 70, 2154–2160. [Google Scholar] [CrossRef] [Green Version]
- Power, J.F.; Willis, W.O.; Grunes, D.L.; Reichman, G.A. Effect of soil temperature, phosphorus and plant age on growth analysis of barley. Agron. J. 1967, 59, 231–234. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. 2018, 252, 72–83. [Google Scholar] [CrossRef]
- Lea, U.S.; Slimestad, R.; Smedvig, P.; Lillo, C. Nitrogen deficiency enhances expression of specific MYB and bHLH transcription factors and accumulation of end products in the flavonoid pathway. Planta 2007, 225, 1245–1253. [Google Scholar] [CrossRef]
- Shapiro, C. Using a chlorophyll meter to manage nitrogen applications to corn with high nitrate irrigation water. Commun. Soil Sci. Plant Anal. 1999, 30, 1037–1049. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Hennig, S.D.; Gnyp, M.L.; Chen, X.; Jia, L.; Bareth, G. Evaluating hyperspectral vegetation indices for estimating nitrogen concentration of winter wheat at different growth stages. Precis. Agric. 2010, 11, 335–357. [Google Scholar] [CrossRef]
- Xia, T.; Miao, Y.; Wu, D.; Shao, H.; Khosla, R.; Mi, G. Active optical sensing of spring corn for in-season diagnosis of nitrogen status based on nitrogen nutrition index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving estimation of summer maize nitrogen status with red edge-based spectral vegetation indices. Field Crops Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Quemada, M.; Alonso-Ayuso, M.; Lizaso, J.; Martín-Lammerding, D. Predicting N status in maize with clip sensors: Choosing sensor, leaf sampling point, and timing. Sensors. 2019, 19, 3881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Tremblay, N.; Zhu, J. A first comparison of Multiplex® for the assessment of corn nitrogen status. J. Food. Agric. Environ. 2012, 10, 1008–1016. [Google Scholar]
- Huang, S.; Miao, Y.; Yuan, F.; Cao, Q.; Ye, H.; Lenz-Wiedemann, V.I.S.; Bareth, G. In-Season diagnosis of rice nitrogen status using proximal fluorescence canopy sensor at different growth stages. Remote Sens. 2019, 11, 1847. [Google Scholar] [CrossRef] [Green Version]
- Varvel, G.E.; Wilhelm, W.W.; Shanahan, J.F.; Schepers, J.S. An algorithm for corn nitrogen recommendations using a chlorophyll meter-based sufficiency index. Agron. J. 2007, 99, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Gong, W.; Shi, S.; Du, L.; Sun, J.; Song, S.; Chen, B.; Zhang, Z. Analyzing the performance of fluorescence parameters in the monitoring of leaf nitrogen content of paddy rice. Sci. Rep. 2016, 6, 28787. [Google Scholar] [CrossRef]
- Agati, G.; Foschi, L.; Grossi, N.; Guglielminetti, L.; Cerovic, Z.G.; Volterrani, M. Fluorescence-based versus reflectance proximal sensing of nitrogen content in Paspalum vaginatum and Zoysia matrella turfgrasses. Eur. J. Agron. 2013, 45, 39–51. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, X.; Ma, Y.; Zhang, R.; Cao, Q.; Zhu, Y.; Cao, W.; Tian, Y. A comparative assessment of measures of leaf nitrogen in rice using two leaf-clip meters. Sensors 2020, 20, 175. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Roca, L.F.; Romero, J.; Bohórquez, J.M.; Alcántara, E.; Fernández-Escobar, R.; Trapero, A. Nitrogen status affects growth, chlorophyll content and infection by Fusicladium oleagineum in olive. Crop Prot. 2018, 109, 80–85. [Google Scholar] [CrossRef]
- Padilla, F.M.; de Souza, R.; Peña-Fleitas, M.T.; Gallardo, M.; Giménez, C.; Thompson, R.B. Different responses of various chlorophyll meters to increasing nitrogen supply in sweet pepper. Front Plant Sci. 2018, 9, 1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obs. 2013, 25, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Bragazza, L.; Freeman, C. High nitrogen availability reduces polyphenol content in Sphagnum peat. Sci. Total Environ. 2007, 377, 439–443. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, D.W.; Liu, D.H.; Geng, M.J.; Zhou, W.B.; Mi, W.J.; Yang, T.W.; Hamilton, D. Influence of nitrogen on the primary and secondary metabolism and synthesis of flavonoids in Chrysanthemum morifolium Ramat. J. Plant Nutr. 2010, 33, 240–254. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhao, Z.; Lei, X.; Xu, X.; Weng, D.; Gao, Y.; Cao, L. Use of fluorescence-based sensors to determine the nitrogen status of paddy rice. J. Agric. Sci. 2013, 151, 862–887. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Proximal optical sensing of cucumber crop N status using chlorophyll fluorescence indices. Eur. J. Agron. 2016, 73, 83–97. [Google Scholar] [CrossRef]
- Cui, Z.L.; Zhang, H.Y.; Chen, X.P.; Zhang, C.C.; Ma, W.Q.; Huang, C.D.; Zhang, W.F.; Mi, G.H.; miAo, Y.X.; Li, X.L.; et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature 2018, 555, 363–366. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Romero, I.; García-Escudero, E.; Martín, I. Leaf blade versus petiole analysis for nutritional diagnosis of Vitis vinifera L. cv. Tempranillo. Am. J. Enol. Vitic. 2012, 64, 50–64. [Google Scholar] [CrossRef]
- Romero, I.; García-Escudero, E.; Martín, I. Effects of leaf position on blade and petiole mineral nutrient concentration of Tempranillo grapevine (Vitis vinifera L.). Am. J. Enol. Vitic. 2010, 61, 544–550. [Google Scholar] [CrossRef] [Green Version]
- Ziadi, N.; Bélanger, G.; Gastal, F.; Claessens, A.; Lemaire, G.; Tremblay, N. Leaf nitrogen concentration as an indicator of corn nitrogen status. Agron. J. 2009, 101, 947–957. [Google Scholar] [CrossRef]
- Kolb, C.A.; Pfündel, E.E. Origins of non-linear and dissimilar relationships between epidermal UV absorbance and UV absorbance of extracted phenolics in leaves of grapevine and barley. Plant Cell Environ. 2005, 28, 580–590. [Google Scholar] [CrossRef]
- Agati, G.; Cerovic, Z.G.; Pinelli, P.; Tattini, M. Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ. Exp. Bot. 2011, 73, 3–9. [Google Scholar] [CrossRef]
- Teal, R.K.; Tubana, B.; Girma, K.; Freeman, K.W.; Arnall, D.B.; Walsh, O.; Raun, W.R. In-season prediction of corn grain yield potential using normalized difference vegetation index. Agron. J. 2006, 98, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Samson, G.; Tremblay, N.; Dudelzak, A.E.; Babichenko, S.M.; Dextraze, L.; Wollring, J. Nutrient stress of corn plants: Early detection and discrimination using a compact multiwavelength fluorescent lidar. In Proceedings of the 4th EARSeL Workshop Lidar Remote Sensing of Land and Sea held during the 20th EARSeL Symposium, Dresden, Germany, 14–16 June 2000. [Google Scholar]
- Overbeck, V.; Schmitz, M.; Tartachnyk, I.; Blanke, M. Identification of light availability in different sweet cherry orchards under cover by using non-destructive measurements with a Dualex™. Eur. J. Agron. 2018, 93, 50–56. [Google Scholar] [CrossRef]
- Padilla, F.M.; Gallardo, M.; Peña-Fleitas, M.T.; De Souza, R.; Thompson, R.B. Proximal optical sensors for nitrogen management of vegetable crops: A review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef] [Green Version]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional roles of flavonoids in photoprotection: New evidence, lessons from the past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Louis, J.; Meyer, S.; Maunoury-Danger, F.; Fresneau, C.; Meudec, E.; Cerovic, Z.G. Seasonal changes in optically assessed epidermal phenolic compounds and chlorophyll contents in leaves of sessile oak (Quercus petraea): Towards signatures of phenological stage. Funct. Plant Biol. 2009, 36, 732–741. [Google Scholar] [CrossRef]
- Louis, J.; Genet, H.; Meyer, S.; Soudani, K.; Montpied, P.; Legout, A.; Dreyer, E.; Cerovic, Z.G.; Dufrêne, E. Tree age-related effects on sun acclimated leaves in a chronosequence of beech (fagus sylvatica) stands. Funct. Plant Biol. 2012, 39, 323–331. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Yang, J.; Wang, H.; Zou, J.; He, J. Effects of nitrogen application rate and leaf age on the distribution pattern of leaf SPAD readings in the rice canopy. PLoS ONE 2014, 9, e92509. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhu, Y.; Jiang, H.; Cao, W. Positional differences in nitrogen and sugar concentrations of upper leaves relate to plant N status in rice under different N rates. Field Crop Res. 2006, 96, 224–234. [Google Scholar] [CrossRef]
- Lin, F.F.; Qiu, L.F.; Deng, J.S.; Shi, Y.Y.; Chen, L.S.; Wang, K. Investigation of SPAD meter-based indices for estimating rice nitrogen status. Compu. Electron. Agric. 2010, 71S, 60–65. [Google Scholar] [CrossRef]
- Abdallah, S.B.; Rabhi, M.; Harbaoui, F.; Zar-kalai, F.; Lachâal, M.; Karray-Bouraoui, N. Distribution of phenolic compounds and antioxidant activity between young and old leaves of Carthamus tinctorius L. and their induction by salt stress. Acta Physiol. Plant. 2013, 35, 1161–1169. [Google Scholar] [CrossRef]
- Vagiri, M.; Conner, S.; Stewart, D.; Andersson, S.C.; Verrall, S.; Johansson, E.; Rumpunen, K. Phenolic compounds in blackcurrant (Ribes nigrum L.) leaves relative to leaf position and harvest date. Food Chem. 2014, 172, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Mulla, D.J.; Miao, Y. Precision Farming. In Land Resources Monitoring, Modeling, and Mapping with Remote Sensing; Thenkabail, P.S., Ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Ziadi, N.; Brassard, M.; Bélanger, G.; Claessens, A.; Tremblay, N.; Cambouris, A.N.; Nolin, M.C.; Parent, L.E. Chlorophyll measurements and nitrogen nutrition index for the evaluation of corn nitrogen status. Agron. J. 2008, 100, 1264–1273. [Google Scholar] [CrossRef]
- Yang, Y.; Timlin, D.J.; Fleisher, D.H.; Lokhande, S.B.; Chun, J.A.; Kim, S.H.; Staver, K.; Reddy, V.R. Nitrogen concentration and dry-matter accumulation in maize crop: Assessing maize nitrogen status with an allometric function and a chlorophyll meter. Commun. Soil Sci. Plant Anal. 2012, 43, 1563–1575. [Google Scholar] [CrossRef]
- Olioso, A.; Méthy, M.; Lacaze, B. Fluorescence as a Function of Canopy Structure and Leaf Fluorescence. Remote. Sens. Environ. 1992, 41, 239–247. [Google Scholar] [CrossRef]
- Garzonio, R.; di Mauro, B.; Colombo, R.; Cogliati, S. Surface reflectance and sun-induced fluorescence spectroscopy measurements using a small hyperspectral UAS. Remote Sens. 2017, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Coppo, P.; Taiti, A.; Pettinato, L.; Francois, M.; Taccola, M.; Drusch, M. Fluorescence imaging spectrometer (FLORIS) for ESA FLEX mission. Remote Sens. 2017, 9, 649. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Mulla, D.J.; Randall, G.W.; Vetsch, J.A.; Vintila, R. Combining chlorophyll meter readings and high spatial resolution remote sensing images for in-season site-specific nitrogen management of corn. Precis. Agric. 2009, 10, 45–62. [Google Scholar] [CrossRef]








| Site | Planting Date | Side Dressing Date | Harvest Date | Irrigation Date | Sampling and Sensing Stage |
|---|---|---|---|---|---|
| 2016 | |||||
| Site 1 | May 7th | Jul. 3rd (57 DAS) | Oct. 6th | Jul. 13–16th (70–73 DAS) | V8 (49 DAS *, 50 DAS), V12 (70 DAS *, 73 DAS), VT (78 DAS *, 81 DAS) |
| Site 2 | May. 5th | Jul. 4th (60 DAS) | Sep. 29th | No irrigation | V8 (50 DAS, 51 DAS *), V13 (72 DAS *), VT (78 DAS, 80 DAS *) |
| 2017 | |||||
| Site 1 | May. 4th | Jul. 3rd (60 DAS) | Oct. 3rd | Jul. 11–13th (69–71 DAS) | V4 (30 DAS *), V6 (40 DAS), V8 (56 DAS), V11 (65 DAS *), VT (84 DAS, 86 DAS *) |
| Site 2 | May. 3th | Jul. 2nd (60 DAS) | Oct. 2nd | No irrigation | V4 (29 DAS *), V6 (38 DAS), V8 (52 DAS), V11 (64 DAS *), VT (83 DAS, 85 DAS *) |
| Parameters | Abbreviation | Algorithm |
|---|---|---|
| Chlorophyll | Chl | FRFR/RFR |
| Flavonoids | Flav | Log (FRFR/FRFUV) |
| Nitrogen balance index | NBI | Chl/Flav |
| Modified chlorophyll | mChl | Chl/DAS |
| Modified flavonoids | mFlav | Flav × DAS |
| Modified nitrogen balance index | mNBI | NBI/DAS2 |
| Dataset | TLNC (g kg−1) | PNC (g kg−1) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | CV (%) | Mean | SD | CV (%) | |
| Calibration dataset | ||||||
| n = 504 | 23.59 | 7.24 | 31 | 20.61 | 8.73 | 42 |
| Validation dataset | ||||||
| n = 252 | 23.08 | 7.07 | 31 | 19.91 | 8.68 | 44 |
| N Concentration (g kg−1) | Leaf Position | Chl | Flav | NBI | mChl | mFlav | mNBI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model | R2 | Model | R2 | Model | R2 | Model | R2 | Model | R2 | Model | R2 | ||
| TLNC | Leaf 1 | Q | 0.34 | Q | 0.11 | P | 0.03 | P | 0.80 | P | 0.53 | P | 0.77 |
| Leaf 2 | Q | 0.34 | Q | 0.16 | P | 0.01 | P | 0.78 | P | 0.49 | P | 0.76 | |
| Leaf 3 | P | 0.18 | Q | 0.10 | Q | 0.01 | P | 0.75 | E | 0.50 | P | 0.74 | |
| PNC | Leaf 1 | Q | 0.20 | Q | 0.22 | Q | 0.03 | Q | 0.84 | P | 0.56 | P | 0.80 |
| Leaf 2 | Q | 0.18 | Q | 0.33 | Q | 0.07 | P | 0.83 | P | 0.49 | P | 0.79 | |
| Leaf 3 | Q | 0.10 | Q | 0.25 | Q | 0.09 | P | 0.78 | P | 0.50 | P | 0.75 | |
| N Concentration (g kg−1) | Leaf Position | mChl | mFlav | mNBI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R2 | RMSE | RE(%) | R2 | RMSE | RE(%) | R2 | RMSE | RE(%) | ||
| TLNC | Leaf 1 | 0.79 | 3.27 | 14 | 0.45 | 5.22 | 23 | 0.72 | 3.76 | 16 |
| Leaf 2 | 0.76 | 3.45 | 15 | 0.42 | 5.38 | 23 | 0.71 | 3.77 | 16 | |
| Leaf 3 | 0.71 | 3.81 | 16 | 0.53 | 4.83 | 21 | 0.73 | 3.65 | 16 | |
| PNC | Leaf 1 | 0.84 | 3.46 | 17 | 0.41 | 6.66 | 33 | 0.73 | 4.52 | 23 |
| Leaf 2 | 0.82 | 3.68 | 18 | 0.40 | 6.73 | 34 | 0.74 | 4.37 | 22 | |
| Leaf 3 | 0.73 | 4.48 | 23 | 0.53 | 5.96 | 30 | 0.75 | 4.29 | 22 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, R.; Miao, Y.; Wang, X.; Chen, Z.; Yuan, F.; Zhang, W.; Li, H. Estimating Plant Nitrogen Concentration of Maize Using a Leaf Fluorescence Sensor across Growth Stages. Remote Sens. 2020, 12, 1139. https://doi.org/10.3390/rs12071139
Dong R, Miao Y, Wang X, Chen Z, Yuan F, Zhang W, Li H. Estimating Plant Nitrogen Concentration of Maize Using a Leaf Fluorescence Sensor across Growth Stages. Remote Sensing. 2020; 12(7):1139. https://doi.org/10.3390/rs12071139
Chicago/Turabian StyleDong, Rui, Yuxin Miao, Xinbing Wang, Zhichao Chen, Fei Yuan, Weina Zhang, and Haigang Li. 2020. "Estimating Plant Nitrogen Concentration of Maize Using a Leaf Fluorescence Sensor across Growth Stages" Remote Sensing 12, no. 7: 1139. https://doi.org/10.3390/rs12071139