Abstract
Purpose
Identifying patients at risk of postoperative complications and trying to prevent these complications are the essence of preoperative evaluation. While not overtly frail or disabled, vulnerable patients with mild frailty may be missed by routine assessments and may still have a worse postoperative course.
Methods
We performed a prospective cohort study evaluating vulnerability in older patients undergoing elective surgery. Vulnerability was assessed using the Clinical Frailty Scale. Our primary outcome was postoperative hospital length of stay (LOS) and our secondary outcome was non-home hospital discharge. We performed multivariable analyses to assess the association between vulnerability and our primary and secondary outcome.
Results
Between 1 January 2017 and 1 January 2018, 271 older patients with a median [interquartile range (IQR)] age of 72 [69–76] yr underwent frailty assessment prior to surgery. Eighty-eight (32.5%) of the cohort were classified as vulnerable. The median [IQR] duration of hospital LOS was 4 [2–7] days for vulnerable patients, 4 [2–6] days for robust patients, and 7 [3–10] days for frail patients. After adjusting for confounders, hospital LOS was not longer for vulnerable patients than for robust patients, but was associated with a higher rate of non-home discharge (odds ratio, 3.7; 95% confidence interval, 1.1 to 12.9; P = 0.04).
Conclusions
Vulnerability was not associated with a longer hospital LOS but with higher risk of non-home discharge. Vulnerable patients might benefit from early identification and advanced planning with earlier transfer to rehabilitation centres.
Résumé
Objectif
L’identification des patients à risque de complications postopératoires et la prévention de ces complications constituent le fondement de l’évaluation préopératoire. Sans être ouvertement fragiles ou handicapés, les patients vulnérables avec une fragilité légère pourraient passer entre les mailles des évaluations de routine et tout de même souffrir d’un parcours postopératoire plus difficile.
Méthode
Nous avons réalisé une étude de cohorte prospective évaluant la vulnérabilité des patients âgés subissant une chirurgie élective. La vulnérabilité a été évaluée à l’aide de l’Échelle Clinical Frailty Scale. Notre critère d’évaluation principal était la durée de séjour hospitalier postopératoire; notre critère d’évaluation secondaire était le congé de l’hôpital sans retour au foyer. Nous avons réalisé des analyses multivariées afin d’évaluer l’association entre la vulnérabilité et nos critères d’évaluation principal et secondaire.
Résultats
Entre le 1er janvier 2017 et le 1er janvier 2018, 271 patients d’un âge médian [écart interquartile (ÉIQ)] de 72 [69–76] ans ont passé une évaluation de fragilité avant leur chirurgie. Quatre-vingt-huit personnes (32,5 %) de la cohorte ont été catégorisées comme vulnérables. La durée médiane [ÉIQ] de séjour hospitalier était de 4 [2–7] jours pour les patients vulnérables, 4 [2–6] pour les patients robustes, et 7 [3–10] pour les patients fragiles. Après l’ajustement pour tenir compte des facteurs confondants, la durée de séjour hospitalier n’était pas plus longue pour les patients vulnérables que pour les patients robustes, mais était associée à un taux plus élevé de congé sans retour au foyer (rapport de cotes, 3,7; intervalle de confiance 95 %, 1,1 à 12,9; P = 0,04).
Conclusion
La vulnérabilité n’a pas été associée à une durée de séjour hospitalier plus longue mais à un risque plus élevé de congé sans retour au foyer. Les patients vulnérables pourraient bénéficier d’une identification précoce et d’une planification avancée avec un transfert plus rapide vers les centres de réadaptation.
Similar content being viewed by others
Identifying patients at risk of postoperative complications and trying to prevent these complications are the essence of preoperative evaluation. With population aging, geriatric concepts such as frailty evaluation has garnered interest in the past decade as a predictor of adverse postoperative outcomes in older surgical patients.1 Frailty is best understood as a loss of reserve due to the accumulation of deficits across multiple physiologic systems, and is often related to multimorbidity and disability.2 Across surgical subspecialties, frail patients face higher risks of mortality, complications, and prolonged hospital stays after their surgical procedure compared with non-frail patients.3,4,5
While some older patients may be overtly frail and more easily recognized as such, surgical risk among others who appear independent but are nonetheless vulnerable, may be underestimated through the failure to routinely apply a validated risk assessment.6 Such patients are in transition from robust to overtly frail, and have been described in the literature as vulnerable.7 Vulnerability, defined as being “slowed up”, while not being dependent, is a transition state between being robust and frail. In contrast, frail patients are characterized by a progressive functional decline in terms of activities and instrumental activities of daily living.8 The Clinical Frailty Scale7 (based on patient’s medical problems and their functional capacity) is categorized into “robust” with a score of 1 to 3, “vulnerable” with a score of 4, and “frail” with a score ≥ 5 (Appendix). Maintaining independence and function and preventing new disability is especially important for older adults after surgery.9,10 In an observational study by Li et al., vulnerable elderly patients undergoing emergent abdominal surgery had a similar odds of 30-day hospital readmission as did frail patients.11
Data are sparse regarding the postoperative course for non-frail vulnerable surgical patients. Therefore, we performed a prospective observational study assessing the impact of vulnerability on the postoperative course in a cohort of seemingly healthy older patients undergoing preoperative assessment prior to elective surgery. We hypothesized that vulnerable older patients would have a longer postoperative hospital length of stay (LOS) and higher prevalence of non-home discharge after surgery compared with robust patients.
Methods
We performed a prospective cohort study in a tertiary academic centre between 1 January 2017 and 1 January 2018, after obtaining approval from our local ethics committee (approved 1 November 2016).
We identified consecutive eligible patients through scheduled daily screening at our hospital’s preoperative clinic. A trained research assistant recruited patients by telephone before their visit. Patients were included if they were ≥ 65 yr old, awaiting major elective orthopedic, general or vascular surgery, and had a planned postoperative stay ≥ 24 hr. We excluded patients undergoing urgent surgery and those unable to understand or provide consent. We also excluded ambulatory surgery, including total knee replacement surgeries (i.e., reserved for fit elderly patients only in our centre).
Our exposure of interest was vulnerability, assessed using the Clinical Frailty Scale scored by a trained research assistant with past experience with this scale.7,12 We chose the Clinical Frailty Scale because it has been associated with adverse outcomes in the surgical setting 8 and it was a better predictor of hospital LOS compared with the FRAIL scale in our previous pilot study.13
We collected demographic variables, comorbidities included in the Charlson Comorbidity Index (CCI) (standardized method of reporting based on a list of 19 comorbidities),14 as well as baseline hemoglobin and creatinine levels as potential confounders. We assessed whether patients underwent general anesthesia or regional anesthesia (defined as spinal anesthesia or regional nerve blocks) as a potential mediator of indirect effect. We assessed the importance of surgery as a potential mediator-outcome confounder. As such, surgical procedures were classified as either major surgery (e.g., intraperitoneal vascular bypass surgery, intraperitoneal gastrointestinal tract surgery, carotid endarterectomy, hyperthermic intraperitoneal chemotherapy) or minor surgery (e.g., limb vascular surgery, hernia repair, ileostomy closure, mastectomy, total knee or hip arthroplasty) (eFigure, available as Electronic Supplementary Material [ESM]). We also assessed whether patients received opioid-based postoperative analgesia (either standard nurse-based analgesia or intravenous patient-controlled analgesia) or any regional analgesia technique (either continuous epidural analgesia or nerve plexus blocks) as well as preoperative American Society of Anesthesiologists physical status score15 and the surgical specialty as descriptive variables.
Our primary outcome was hospital LOS. Our secondary outcome was worsening of functional impairment in the postoperative period defined as non-home discharge to a post-acute care facility after surgery (defined as a rehabilitation centre, a senior residence, or a long-term care facility). Since patients could have been residing in such settings before admission, only a deterioration in disposition status was included. Importantly, no patients in our cohort were residing in long-term care facilities before surgery (Table 1) and there was no planned discharged to rehabilitation centre or senior residence for any patient prior to surgery. We chose the outcome of discharge to a post-acute care facility because past studies have shown that it is a significant indicator of functional decline among older persons,16 and is associated with increased postoperative mortality17 and increased cost of care.18
A research assistant contacted eligible patients by telephone and performed a semi-structured interview to assess their preoperative frailty state. This research assistant did not participate in other data collection and was not in a position to influence clinical practice. We chose this method to facilitate patient recruitment and to avoid extra visits to the preoperative clinic or prolonging a planned visit. Telephone-based frailty assessment has previously been shown to be feasible in the preoperative setting.13 Past study has shown that the telephone-based Clinical Frailty Scale had adequate agreement with in-person physician assessment (kappa = 0.69).19 In our pilot study in an orthopedic surgical population, a frail state derived from a telephone-based Clinical Frailty Scale was feasible and was an independent predictor of a longer hospital LOS compared with robust patients.13 While no measurement validity of a telephone-based scoring compared with an in-person scoring was available, we believed telephone-based scoring might be more conservative (more patients classified as robust vs vulnerable) because the patient’s physical status was not seen (slow gait speed and cachexia for example). Other research assistants unaware of patients’ frailty status collected baseline demographic variables and other covariables through chart review using standardized report forms.
Statistical analyses
Few studies have evaluated the effect of vulnerability on hospital LOS in a cohort of general surgery patients. We estimated our sample size based on results from our pilot study. We planned on recruiting a convenience sample of 300 patients, which was the estimated number of eligible patients over a one-year period. Using an estimated baseline LOS of five days in robust patients, a two-sided alpha of 0.05, a risk ratio for vulnerable patients of 1.2, an estimated proportion of vulnerable patients as 40% of our sample, and an r2 of our covariables of 0.1, our sample provided a power of 93%.
We reported all categorical variables as proportions and continuous variables as mean (standard deviation [SD]) or median [interquartile range (IQR)] for skewed distributions after assessment of normal distribution using the Shapiro–Wilk test. We compared categorical variables using Chi-Square tests and continuous variables using either one-way analysis of variance or Kruskal–Wallis one-way analysis of variance between our three groups (robust, vulnerable, and frail).
For our primary outcome, we performed a prespecified multivariable negative binomial regression to assess the controlled direct effect of vulnerability on hospital LOS, robust patients being the reference group. We used a negative binomial regression rather than a Poisson regression to fit distribution dispersion. We chose covariates for the model based on an epidemiological conceptual model (eFigure as ESM). We included covariates if they were potential confounders of the association between frailty and hospital LOS (i.e., age, sex, CCI, preoperative hemoglobin and creatinine concentrations may have an effect on both the frailty level and the LOS), a mediator of an indirect effect of frailty on our outcomes (choice of intraoperative anesthesia technique depends on anesthesiologist’s assessment and might affect LOS), or a confounder of the mediator-outcome association (major or minor surgical procedures influence anesthesia choice and will affect LOS). Associations between covariates and LOS were reported as risk multiplication factors (RMF), which stands for the exponential fitted coefficient of the negative binomial regression model. As an example, an RMF of 1.5 for vulnerable patients can be translated to vulnerable patients having 1.5 times the hospital LOS of robust patients. For the disposition to discharge, we performed a prespecified multivariable logistic regression model in a similar fashion with robust patients being the reference group. We used asymptotic standard errors for all estimates. Collinearity between our independent variables was evaluated by calculating tolerance and variance inflation factor values.
As a sensitivity analysis, to test our hypothesis and identify vulnerable patients without apparent significant functional impairment, we also included patients with a Clinical Frailty Scale score of 3 as vulnerable. These patients are defined as having their medical problems under control and not interfering with regular activities. We hypothesized that some patients might not self-report fatigue (or being slowed up) and thus be categorized as a score of 3. Furthermore, this definition of vulnerability (Clinical Frailty Scale score of 3 and 4) has been used previously.11,20 Even though patients with a score of 5 are only mildly frail, they still have some form of functional impairment and were not included as vulnerable in sensitivity analyses.
Results
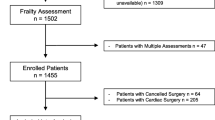
Of the 743 patients eligible, 302 patients were included (Figure). Of those, 30 did not receive the intended operation: two died prior to surgery, five were deemed inoperable because of high surgical risk, and nine were still awaiting surgery at study end. Surgery was performed on 272 patients and two patients removed their consent after surgery, leaving a total of 270 patients in our final analyses (Table 1). The median [IQR] age was 72 [69–76] yr and 53.3% (n = 144) of our cohort were female. The median [IQR] CCI score was 4 [3–6]. Thirty-one (11.5%) of patients had an active cancer. Most of our patients lived at home prior to surgery (92.6%, n = 250). Orthopedic procedures were represented by 61.9% (n = 167) of our cohort, followed by general surgery (24.4%, n = 66), and vascular surgery (13.7%, n = 37).
Using the Clinical Frailty Scale, 51.1% (n = 138) of our cohort was classified as robust, 32.2% (n = 87) as vulnerable, and 16.6% (n = 45) as frail (Table 1). Among frail patients, 44 patients were lightly frail, one patient was moderately frail (score = 6), and no patient was classified as severely frail (score ≥ 7). Vulnerable and frail patients were more frequently female than robust patients were (P = 0.02) and vulnerable patients had a higher prevalence of chronic obstructive pulmonary disease (21.6%, n = 19) than robust (8.7%, n = 12) and frail patients did (15.6%, n = 7) (P = 0.01) (Table 1). The method of intraoperative anesthesia and postoperative analgesia did not differ significantly between vulnerable, frail, and robust patients (Table 1).
During follow-up, one patient died during hospitalization. He was classified as vulnerable and underwent abdominal surgery. Of the 269 survivors, the median [IQR] duration of hospital LOS was four [2–7] days for vulnerable patients, four [2–6] days for robust patients, and seven [3–10] days for frail patients (P = 0.02). The Clinical Frailty Scale had a significant controlled direct effect on hospital LOS after adjusting for confounders (Table 3). Frail patients were more likely to be hospitalized for a longer duration than robust patients were (RMF, 1.88; 95% confidence interval [CI], 1.27 to 2.79; P = 0.002). This association was not significant for vulnerable patients (RMF, 1.29; 95% CI, 0.93 to 1.77; P = 0.13).
In terms of disposition, 82.2% were discharged home (n = 221), while 11.2% (n = 30) were discharged to a rehabilitation centre and 6.7% (n = 18) to a senior residence (Table 2). Eleven (14.8%) of the vulnerable patients had a new transfer to a post-acute care facility compared with four (2.9%) of robust patients and 16 (35.6%) of frail patients. These transfers were all to a rehabilitation facility except one transfer of a frail patient to a senior residency. In our multivariable logistic regression model, the Clinical Frailty Scale had also a significant controlled direct effect on our secondary outcome (Table 4). Vulnerable patients (odds ratio [OR], 3.76; 95% CI, 1.13 to 12.88; P = 0.04) or frail patients (OR, 13.09; 95% CI, 3.82 to 45.08; P < 0.001) had a higher odds than robust patients of a new transfer to post-acute care facilities.
Our sensitivity analysis reclassified 83 patients with a Clinical Frailty Scale score of 3 from robust to vulnerable, increasing the proportion of vulnerable patients to 63.0% (n = 170). Being vulnerable was not significantly associated with hospital LOS nor a new discharge to post-acute care facility using this new definition (see ESM; eTable 1 and eTable 2). There was no significant collinearity between Clinical Frailty Scale and other covariates in both our models.
Discussion
Even in a seemingly healthy group of older patients, a vulnerable state was present in one third of our patients. Vulnerable patients did not have a longer hospital LOS compared with robust patients but were more often discharged to post-acute care facilities. The importance of surgery was itself associated with a longer hospital LOS but not with discharge to post-acute care facilities.
A similar study in a cohort of 1,215 patients undergoing transcatheter aortic valve replacement surgery identified 32.9% of their cohort as being vulnerable.21 Although similar in number, their cohort was much older with a mean (SD) age of 84 (5) yr compared with our mean [IQR] age of 72 [69–76] yr. When we included patients with a Clinical Frailty Scale of 3 in our definition, the prevalence of vulnerability doubled, and was higher than that reported by Li et al. in an emergent surgical cohort.11 Similarly, readmission and deaths were significantly associated with the Clinical Frailty Scale in both studies.
We did not find an association between vulnerability and a longer hospital LOS with a direct controlled effect model. Since one important factor associated with hospital LOS is the presence of disability before surgery,22 this might explain why LOS was similar between robust and vulnerable patients in our cohort. Postoperative programs, such as Enhanced Recovery After Surgery,23 targeting early mobilization have already been implemented in our institution in abdominal surgical patients. This might lead to a faster recovery time of vulnerable patients and a faster identification of those benefiting from a transfer to rehabilitation centre instead of completing their physiotherapy program in-hospital. Such an effect might be taken into account in our controlled direct effect model through the indirect effect mediated by the type of anesthesia and type of surgery. Nevertheless, being frail was associated with a longer hospital LOS. We already know that frail patients are at higher risk of postoperative complications such as delirium, infection, and intensive care unit admissions.1 These factors might explain the longer LOS among frail patients, but we did not measure them in our study.
Being vulnerable was independently associated with a higher odds of discharge to rehabilitation centres that being robust was, with the frailty level having a dose-response effect on this outcome. This suggests that an important subset of functionally independent and medically stable older patients is indeed at increased risk of adverse postoperative outcomes and a postoperative decrease in autonomy notwithstanding the effect of surgery. Similarly, using the National Surgical Quality Improvement Program database, a study showed that pre-frail patients undergoing radical cystectomy were less likely to return home after their intervention (adjusted OR, 1.37; 95% CI, 1.07 to 1.74).24 Most of our patients not discharged home were transferred to rehabilitation centres. While they would probably be discharged home afterward, being able to identify these patients earlier on can improve coordination, plan preoperative rehabilitation programs, diminish transfer delays, and improve healthcare services.
Preoperative rehabilitation programs are not part of the routine patient management in our centre and their applicability and efficacy in managing different components of frailty remain unclear. A study on cardiac surgery patients showed improved preoperative frailty state after preoperative rehabilitation, although change in postoperative trajectories were not assessed.25 Furthermore, interventions in community-dwelling older populations suggest physical therapy programs may require as long as six months to have an impact on physical function,26 limiting their applicability in surgical settings. Vulnerable patients might better respond to shorter duration and focused preoperative interventions, as well as adapted in-hospital interventions such as anesthetic approach, fluid management, and delirium preventions.
Our study has many strengths. First, we were able to show a positive association between vulnerability and adverse outcomes in a healthy subgroup of elderly patients. Also, our recruitment process and assessment methods minimized extra time spent at the preoperative clinic, allowing for easier consent process and better overall feasibility for future multicentred studies. Finally, we were able to uniquely examine the direct effect of preoperative frailty on patient outcomes without the effect of the intraoperative anesthetic technique and type of surgery.
There are limitations to our study. By only including elective patients, we had a skewed distribution with few people in the highest frailty categories since they might be considered at too high risk for surgery. While we conducted a direct controlled effect analysis that excluded the effect of type of anesthesia and severity of surgery on the postoperative course, our model probably did not take into account all variables on the complex indirect causal pathway between frailty and postoperative trajectory. Many unmeasured confounders might also have biased our observations and we cannot exclude residual confounding. Also, compared with our sample size calculation, our median LOS was shorter and the proportion of vulnerable patients was also lower. These might have resulted in a lower than expected power, as suggested by our large 95% CI. Even though the observed RMF from our primary analysis of 1.29 may suggest an effect, random error is highly possible, and a more powerful study might find different results. Another limitation is that we performed a single-centred study limited to some surgical subspecialties; we did not include patients from other specialties (e.g., neurosurgery, thoracic, and urogenital surgery) who are not seen on a statutory basis in our preoperative clinic. This might have biased the observed effect, since orthopedic and non-oncologic general surgeries are generally performed on more robust patients. Finally, a significant number of patients could not be contacted on time before surgery. This may limit the generalizability of our results to other surgical settings and populations.
In conclusion, vulnerable older surgical patients are at higher risk for requiring postoperative rehabilitation facilities but did not seem to have a longer hospital LOS. Identifying these patients beforehand may help healthcare professionals optimize the patient’s transition between acute and post-acute care.
References
Lin HS, Watts JN, Peel NM, Hubbard RE. Frailty and post-operative outcomes in older surgical patients: a systematic review. BMC Geriatr 2016; DOI: https://doi.org/10.1186/s12877-016-0329-8.
De Lepeleire J, Iliffe S, Mann E, Degryse JM. Frailty: an emerging concept for general practice. Br J Gen Pract 2009; 59: e177-82.
Afilalo J, Mottillo S, Eisenberg MJ, et al. Addition of frailty and disability to cardiac surgery risk scores identifies elderly patients at high risk of mortality or major morbidity. Circ Cardiovasc Qual Outcomes 2012; 5: 222-8.
Donald GW, Ghaffarian AA, Isaac F, et al. Preoperative frailty assessment predicts loss of independence after vascular surgery. J Vasc Surg 2018; 68: 1382-9.
Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210: 901-8.
Ahmed A, Sorajja P, Pai A, et al. Prospective evaluation of the eyeball test for assessing frailty in patients with valvular heart disease. J Am Coll Cardiol 2016; 68: 2911-2.
Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005; 173: 489-95.
Okabe H, Ohsaki T, Ogawa K, et al. Frailty predicts severe postoperative complications after elective colorectal surgery. Am J Surg 2019; 217: 677-81.
Brinson Z, Tang VL, Finlayson E. Postoperative functional outcomes in older adults. Curr Surg Rep 2016; DOI: https://doi.org/10.1007/s40137-016-0140-7.
McIsaac DI, Jen T, Mookerji N, Patel A, Lalu MM. Interventions to improve the outcomes of frail people having surgery: a systematic review. PLoS One 2017; DOI: https://doi.org/10.1371/journal.pone.0190071.
Li Y, Pederson JL, Churchill TA, et al. Impact of frailty on outcomes after discharge in older surgical patients: a prospective cohort study. CMAJ 2018; 190: E184-90.
Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001; 1: 323-36.
Wang HT, Fafard J, Ahern S, Vendittoli PA, Hebert P. Frailty as a predictor of hospital length of stay after elective total joint replacements in elderly patients. BMC Musculoskelet Disord 2018; . https://doi.org/10.1186/s12891-018-1935-8.
Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45: 613-9.
Owens WD, Felts JA, Spitznagel EL Jr. ASA physical status classifications: a study of consistency of ratings. Anesthesiology 1978; 49: 239-43.
Balentine CJ, Naik AD, Berger DH, Chen H, Anaya DA, Kennedy GD. Postacute care after major abdominal surgery in elderly patients: intersection of age, functional status, and postoperative complications. JAMA 2016; 151: 759-66.
Legner VJ, Massarweh NN, Symons RG, McCormick WC, Flum DR. The significance of discharge to skilled care after abdominopelvic surgery in older adults. Ann Surg 2009; 249: 250-5.
Mechanic R. Post-acute care–the next frontier for controlling Medicare spending. N Engl J Med 2014; 370: 692-4.
Chan DC, Tsou HH, Chen CY, Chen CY. Validation of the Chinese-Canadian study of health and aging clinical frailty scale (CSHA-CFS) telephone version. Arch Gerontol Geriatr 2010; 50: e74-80.
Reichart D, Rosato S, Nammas W, et al. Clinical frailty scale and outcome after coronary artery bypass grafting. Eur J Cardiothorac Surg 2018; 54: 1102-9.
Shimura T, Yamamoto M, Kano S, et al. Impact of the clinical frailty scale on outcomes after transcatheter aortic valve replacement. Circulation 2017; 135: 2013-24.
Collins TC, Daley J, Henderson WH, Khuri SF. Risk factors for prolonged length of stay after major elective surgery. Ann Surg 1999; 230: 251-9.
Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg 2017; 152: 292-8.
Pearl JA, Patil D, Filson CP, et al. Patient frailty and discharge disposition following radical cystectomy. Clin Genitourin Cancer 2017; 15: e615-21.
Waite I, Deshpande R, Baghai M, Massey T, Wendler O, Greenwood S. Home-based preoperative rehabilitation (prehab) to improve physical function and reduce hospital length of stay for frail patients undergoing coronary artery bypass graft and valve surgery. J Cardiothorac Surg 2017; DOI: https://doi.org/10.1186/s13019-017-0655-8.
Tarazona-Santabalbina FJ, Gomez-Cabrera MC, Perez-Ros P, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc 2016; 17: 426-33.
Author contributions
Han Ting Wang was involved in all aspects of this study. François Martin Carrier performed data analysis, manuscript writing, and revision. Anne Tremblay performed data collection, manuscript writing, and revision. Marie-Maude Joly performed data collection, manuscript writing, and revision. Rafik Ghali was involved in study design and protocol writing, overseeing project completion, and manuscript revision. George Heckman, John P. Hirdes, and Paul Hebert were involved in study design, protocol writing, manuscript writing, and revision. All authors contributed significantly and met requirements for authorship.
Conflicts of interest
None.
Funding statement
This work was supported by research grant obtained from the Canadian Frailty Network (Strategic Impact Grant: SIG2014F-31).
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix
Appendix
Description of individual score of the Clinical Frailty Scale
Score | Clinical description |
|---|---|
1 | Very fit—robust, energetic, and they usually exercise regularly |
2 | Well—no active disease symptoms, they are active occasionally |
3 | Managing well—medical conditions are under control, not regularly active |
4 | Vulnerable—While not dependent, common complaint is being “slowed up” |
5 | Mildly frail—more evident slowing, and need help for higher order IADLs (finance, transportation for example) |
6 | Moderately frail—dependent for all IADLs and also often with stairs and bathing |
7 | Severely frail—completely dependent for personal care |
8 | Very severely frail—completely dependent and approaching end of life |
Rights and permissions
About this article
Cite this article
Wang, H.T., Carrier, F.M., Tremblay, A. et al. Outcomes of vulnerable elderly patients undergoing elective major surgery: a prospective cohort study. Can J Anesth/J Can Anesth 67, 847–856 (2020). https://doi.org/10.1007/s12630-020-01646-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-020-01646-z