Abstract
Background
Hypozincaemia may develop in critically ill patients, including those with acute brain injury in the early phase after hospital admission. The aim of this study was to investigate the prevalence of hypozincaemia after aneurysmal subarachnoid haemorrhage (aSAH) and its association with delayed cerebral ischemia and functional outcome.
Methods
We retrospectively analysed a cohort of 384 patients with SAH admitted to the Neurointensive Care Unit at Rigshospitalet, Copenhagen, Denmark, in whom at least one measurement of plasma zinc concentration was done during the hospital stay. Hypozincaemia was defined as at least one measurement of plasma zinc below 10 μmol/L. Potential associations between hypozincaemia, demographic variables and functional outcome after aSAH were analysed in multivariable logistic regression models.
Results
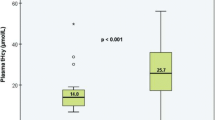
Hypozincaemia was observed in 67% (n = 257) of all patients and occurred within 7 days in more than 95% of all hypozincaemic patients. In a multivariable model, severe SAH (WFNS 3–5; OR 4.2, CI 2.21–8.32, p < 0.001) and Sequential Organ Failure Assessment (SOFA) score on the day of admission (OR 1.24, CI 1.11–1.40, p < 0.001) were independently associated with hypozincaemia. In another multivariable model, hypozincaemia was independently associated with an unfavourable outcome (defined as a modified Rankin Scale score from 3 to 6) (OR 1.97, CI 1.06–3.68, p = 0.032), as was age (OR 1.03, CI 1.01–1.05, p = 0.015), SOFA score on the day of admission (OR 1.14, CI 1.02–1.29, p = 0.02), a diagnosis of delayed cerebral ischaemia (OR 4.06, CI 2.29–7.31, p < 0.001) and a clinical state precluding assessment for delayed cerebral ischaemia (OR 15.13, CI 6.59–38.03, p < 0.001).
Conclusion
Hypozincaemia occurs frequently after aSAH, is associated with a higher disease severity and independently contributes to an unfavourable outcome.



Similar content being viewed by others

Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
- aSAH:
-
Aneurysmal subarachnoid haemorrhage
- SOFA:
-
Sequential Organ Failure Assessment
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- OR:
-
Odds ratio
- mRS:
-
Modified Rankin Scale
- DCI:
-
Delayed cerebral ischaemia
- NICU:
-
Neurointensive Care Unit
- WFNS:
-
World Federation of Neurosurgical Societies
- GCS:
-
Glasgow Coma Scale
References
Beale RJ, Sherry T, Lei K, Campbell-Stephen L, McCook J, Smith J, Venetz W, Alteheld B, Stehle P, Schneider H (2008) Early enteral supplementation with key pharmaconutrients improves Sequential Organ Failure Assessment score in critically ill patients with sepsis: outcome of a randomized, controlled, double-blind trial. Crit Care Med 36:131–144. https://doi.org/10.1097/01.CCM.0000297954.45251.A9
Berger MM, Soguel L, Shenkin A, Revelly J-P, Pinget C, Baines M, Chioléro RL (2008) Influence of early antioxidant supplements on clinical evolution and organ function in critically ill cardiac surgery, major trauma, and subarachnoid hemorrhage patients. Crit Care 12. https://doi.org/10.1186/cc6981
Berger MM, Spertini F, Shenkin A, Wardle C, Wiesner L, Schindler C, Chiolero RL (1998) Trace element supplementation modulates pulmonary infection rates after major burns: a double-blind, placebo-controlled trial. Am J Clin Nutr 68:365–371. https://doi.org/10.1093/ajcn/68.2.365
Besecker BY, Exline MC, Hollyfield J, Phillips G, DiSilvestro RA, Wewers MD, Knoell DL (2011) A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr 93:1356–1364. https://doi.org/10.3945/ajcn.110.008417
Bonaventura P, Benedetti G, Albarède F, Miossec P (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14:277–285. https://doi.org/10.1016/j.autrev.2014.11.008
Boosalis MG, Solem LD, McCall JT, Ahrenholz DH, McClain CJ (1988) Serum zinc response in thermal injury. J Am Coll Nutr 7:69–76. https://doi.org/10.1080/07315724.1988.10720222
Briassoulis G, Filippou O, Hatzi E, Papassotiriou I, Hatzis T (2005) Early enteral administration of immunonutrition in critically ill children: results of a blinded randomized controlled clinical trial. Nutrition 21:799–807. https://doi.org/10.1016/j.nut.2004.12.006
Cander B, Dundar ZD, Gul M, Girisgin S (2011) Prognostic value of serum zinc levels in critically ill patients. J Crit Care 26:42–46. https://doi.org/10.1016/j.jcrc.2010.06.002
Choi DW, Koh JY (1998) Zinc and brain injury. Annu Rev Neurosci 21:347–375. https://doi.org/10.1146/annurev.neuro.21.1.347
Cirino Ruocco MA, Pacheco Cechinatti ED, Barbosa F, Navarro AM (2018) Zinc and selenium status in critically ill patients according to severity stratification. Nutrition 45:85–89. https://doi.org/10.1016/j.nut.2017.07.009
Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P, American Heart Association Stroke Council, Council on Cardiovascular Radiology and Intervention, Council on Cardiovascular Nursing, Council on Cardiovascular Surgery and Anesthesia, Council on Clinical Cardiology (2012) Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 43:1711–1737. https://doi.org/10.1161/STR.0b013e3182587839
Florea D, Molina-López J, Hogstrand C, Lengyel I, de la Cruz AP, Rodríguez-Elvira M, Planells E (2018) Changes in zinc status and zinc transporters expression in whole blood of patients with Systemic Inflammatory Response Syndrome (SIRS). J Trace Elem Med Biol 49:202–209. https://doi.org/10.1016/j.jtemb.2017.11.013
Foster M, Samman S (2012) Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients 4:676–694. https://doi.org/10.3390/nu4070676
Gower-Winter SD, Levenson CW (2012) Zinc in the central nervous system: from molecules to behavior. BioFactors 38:186–193. https://doi.org/10.1002/biof.1012
Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG (2013) A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 368:1489–1498. https://doi.org/10.1097/01.sa.0000441043.83064.4c
Heyland DK, Jones N, Cvijanovich NZ, Wong H (2008) Zinc supplementation in critically ill patients: a key pharmaconutrient? JPEN J Parenter Enteral Nutr 32:509–519. https://doi.org/10.1177/0148607108322402
Jang JY, Shim H, Lee SH, Lee JG (2014) Serum selenium and zinc levels in critically ill surgical patients. J Crit Care 29:317.e5–317.e8. https://doi.org/10.1016/j.jcrc.2013.12.003
King JC, Shames DM, Woodhouse LR (2000) Zinc homeostasis in humans. J Nutr 130:1360S–1366S. https://doi.org/10.1093/jn/130.5.1360S
Krishna C, Sonig A, Natarajan SK, Siddiqui AH (2014) The expanding realm of endovascular neurosurgery: flow diversion for cerebral aneurysm management. Methodist Debakey Cardiovasc J 10:214–219. https://doi.org/10.14797/mdcj-10-4-214
Lee YH, Bang ES, Lee JH, Lee JD, Kang DR, Hong J, Lee JM (2019) Serum concentrations of trace elements zinc, copper, selenium, and manganese in critically ill patients. Biol Trace Elem Res 188:316–325. https://doi.org/10.1007/s12011-018-1429-4
Lees KR, Selim MH, Molina CA, Broderick JP (2016) Early versus late assessment of stroke outcome. Stroke 47:1416–1419. https://doi.org/10.1161/STROKEAHA.115.011153
Lin CN, Howng SL, Hu SH, Huang TJ (1992) Assessments of nutritional status and immunological responses in head trauma: alterations in zinc and C-reactive protein. Kaohsiung J Med Sci 8:195–201
Linko R, Karlsson S, Pettilã V, Varpula T, Okkonen M, Lund V, Ala-Kokko T, Ruokonen E (2011) Serum zinc in critically ill adult patients with acute respiratory failure. Acta Anaesthesiol Scand 55:615–621. https://doi.org/10.1111/j.1399-6576.2011.02425.x
Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ (2005) Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci 102:6843–6848. https://doi.org/10.1073/pnas.0502257102
McClain CJ, Twyman DL, Ott LG, Rapp RP, Tibbs PA, Norton JA, Kasarskis EJ, Dempsey RJ, Young B (1986) Serum and urine zinc response in head-injured patients. J Neurosurg 64:224–230. https://doi.org/10.3171/jns.1986.64.2.0224
Morris DR, Levenson CW (2013) Zinc in traumatic brain injury: from neuroprotection to neurotoxicity. Curr Opin Clin Nutr Metab Care 16:708–711. https://doi.org/10.1097/MCO.0b013e328364f39c
Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ (2009) Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 8:635–642. https://doi.org/10.1016/S1474-4422(09)70126-7
Olsen MH, Orre M, Leisner ACW, Rasmussen R, Bache S, Welling K-L, Eskesen V, Møller K (2019) Delayed cerebral ischaemia (DCI) in patients with aneurysmal subarachnoid haemorrhage: functional outcome and long-term mortality. Acta Anaesthesiol Scand 63:1191–1199. https://doi.org/10.1111/aas.13412
Ott L, Mcclain CJ, Gillespie M, Young B (1994) Cytokines and metabolie dysfunction after severe head injury. J Neurotrauma 11:447–472. https://doi.org/10.1089/neu.1994.11.447
Ott L, Young B, McClain C (1987) The metabolic response to brain injury. J Parenter Enter Nutr 11:488–493. https://doi.org/10.1177/0148607187011005488
Pankhurst MW, Gell DA, Butler CW, Kirkcaldie MTK, West AK, Chung RS (2012) Metallothionein (MT) -I and MT-II expression are induced and cause zinc sequestration in the liver after brain injury. PLoS One 7:e31185. https://doi.org/10.1371/journal.pone.0031185
Rosenørn J, Eskesen V (1994) Patients with ruptured intracranial saccular aneurysms: clinical features and outcome according to the size. Br J Neurosurg 8:73–78. https://doi.org/10.3109/02688699409002396
Rosenørn J, Eskesen V, Schmidt K, Rønde F (1987) The risk of rebleeding from ruptured intracranial aneurysms. J Neurosurg 67:329–332. https://doi.org/10.3171/jns.1987.67.3.0329
Sensi SL, Paoletti P, Koh J-Y, Aizenmann E, Bush AI, Hershfinkel M (2011) The neurophysiology and pathology of brain zinc. J Neurosci 31:16076–16085. https://doi.org/10.1016/j.micinf.2011.07.011.Innate
Vergouwen MDI, Vermeulen M, van Gijn J, Rinkel GJE, Wijdicks EF, Muizelaar JP, Mendelow AD, Juvela S, Yonas H, Terbrugge KG, Macdonald RL, Diringer MN, Broderick JP, Dreier JP, Roos YBWEM (2010) Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke 41:2391–2395. https://doi.org/10.1161/STROKEAHA.110.589275
Wang Y, Su R, Lv G, Cao Y, Fan Z, Wang Y, Zhang L, Yu D, Mei X (2014) Supplement moderate zinc as an effective treatment for spinal cord injury. Brain Res 1545:45–53. https://doi.org/10.1016/j.brainres.2013.12.015
Wong HR, Shanley TP, Sakthivel B, Cvijanovich N, Lin R, Allen GL, Thomas NJ, Doctor A, Kalyanaraman M, Tofil NM, Penfil S, Monaco M, Tagavilla MA, Odoms K, Dunsmore K, Barnes M, Aronow BJ (2007) Genome-level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics 30:146–155. https://doi.org/10.1152/physiolgenomics.00024.2007
Young AB, Ott LG, Beard D, Dempsey RJ, Tibbs PA, McClain CJ (1988) The acute-phase response of the brain-injured patient. J Neurosurg 69:375–380. https://doi.org/10.3171/jns.1988.69.3.0375
Young B, Ott L, Kasarskis E, Rapp R, Moles K, Dempsey RJ, Tibbs PA, Kryscio R, McClain C (1996) Zinc supplementation is associated with improved neurologic recovery rate and visceral protein levels of patients with severe closed head injury. J Neurotrauma 13:25–34. https://doi.org/10.1089/neu.1996.13.25
Acknowledgements
We would like to thank bioinformatician Jesper Qvist Thomassen, from the Department of Clinical Biochemistry Rigshospitalet, for his assistance regarding data extraction.
Authors’ contribution statements
TA, MHO, RFS and KM conceived the idea of the project. MO, RR, SB, VE and RFS delivered the data available. TA and MHO carried out the analytical methods. TA wrote the manuscript and was supervised by MHO and KM. All authors discussed the results and contributed to the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was performed by data extraction and analysis from patient charts, and therefore, for this type of study, approval by a Committee on Health Research Ethics and formal consent from patients or relatives was not required according to Danish law.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurosurgical intensive care
Rights and permissions
About this article
Cite this article
Arleth, T., Olsen, M.H., Orre, M. et al. Hypozincaemia is associated with severity of aneurysmal subarachnoid haemorrhage: a retrospective cohort study. Acta Neurochir 162, 1417–1424 (2020). https://doi.org/10.1007/s00701-020-04310-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-020-04310-z