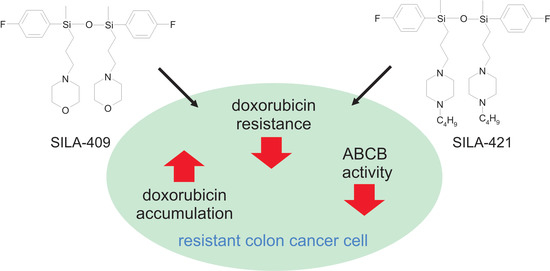
Organosilicon Compounds, SILA-409 and SILA-421, as Doxorubicin Resistance-Reversing Agents in Human Colon Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity of Disiloxanes
2.2. Influence of Disiloxanes on Doxorubicin Cytotoxicity
2.3. Intracellular Accumulation of Rhodamine 123 (R123)
2.4. Intracellular Accumulation of Doxorubicin
2.5. Expression of ABCB1 Transporter
3. Materials and Methods
3.1. Chemicals
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Isobolographic Analysis
3.5. Accumulation of Rhodamine 123 in Cancer Cells
3.6. Intracellular Accumulation of Doxorubicin
3.7. Expression of ABCB1 Protein
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gottesman, M.M. Mechanisms of cancer drug resistance. Ann. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.H.; Chen, Z.S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Bidet, M.; Tomico, A.; Martin, P.; Guizouarn, H.; Mollat, P.; Mus-Veteau, I. The Hedgehog receptor patched functions in multidrug transport and chemotherapy resistance. Mol. Cancer Res. 2012, 10, 1496–1508. [Google Scholar] [CrossRef] [Green Version]
- Hasanovic, A.; Ruggiero, C.; Jung, S.; Rapa, I.; Signetti, L.; Ben Hadj, M.; Terzolo, M.; Beuschlein, F.; Volante, M.; Hantel, C.; et al. Targeting the multidrug transporter Patched potentiates chemotherapy efficiency on adrenocortical carcinoma in vitro and in vivo. Int. J. Cancer 2018, 143, 199–211. [Google Scholar] [CrossRef] [Green Version]
- Sharom, F.J. ABC multidrug transporters: Structure, function and role in chemoresistance. Pharmacogenomics 2008, 9, 105–127. [Google Scholar] [CrossRef]
- Shukla, S.; Wu, C.P.; Ambudkar, S.V. Development of inhibitors of ATP-binding cassette drug transporters: Present status and challenges. Expert Opin. Drug Metab. Toxicol. 2008, 4, 205–223. [Google Scholar] [CrossRef]
- Kelly, R.J.; Draper, D.; Chen, C.C.; Robey, R.W.; Figg, W.D.; Piekarz, R.L.; Chen, X.; Gardner, E.R.; Balis, F.M.; Venkatesan, A.M.; et al. A pharmacodynamic study of docetaxel in combination with the P-glycoprotein antagonist tariquidar (XR9576) in patients with lung, ovarian, and cervical cancer. Clin. Cancer Res. 2011, 17, 569–580. [Google Scholar] [CrossRef] [Green Version]
- Cripe, L.D.; Uno, H.; Paietta, E.M.; Litzow, M.R.; Ketterling, R.P.; Bennett, J.M.; Rowe, J.M.; Lazarus, H.M.; Luger, S.; Tallman, M.S. Zosuquidar, a novel modulator of P-glycoprotein, does not improve the outcome of older patients with newly diagnosed acute myeloid leukemia: A randomized, placebo-controlled trial of the Eastern Cooperative Oncology Group 3999. Blood 2010, 116, 4077–4085. [Google Scholar] [CrossRef]
- Sekhon, B.S. Metalloid compounds as drugs. Res. Pharm. Sci. 2013, 8, 145–158. [Google Scholar] [PubMed]
- Gately, S.; West, R. Novel therapeutics with enhanced biological activity generated by the strategic introduction of silicon isosteres into known drug scaffolds. Drug Develop. Res. 2007, 68, 156–163. [Google Scholar] [CrossRef]
- Mousazadeh, H.; Milani, M.; Zarghami, N.; Alizadeh, E.; Safa, K.D. Study of the cytotoxic and bactericidal effects of sila-substituted thioalkyne and mercapto-thione compounds based on 1,2,3-triazole scaffold. Basic Clin. Pharmacol Toxicol. 2017, 121, 390–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, A.; Hegyes, P.; Molnar, J.; Mucsi, I.; Hever, A.; Szabo, D.; Kiesig, S.; Lage, H.; Gaal, D.; Nacsa, J. Substituted Disiloxanes, Method for the Production Thereof and the Use Thereof for Reversal of Multidrug Resistance (MDR). EP Patent 1 432 717 B1, 19 May 1999. [Google Scholar]
- Molnar, J.; Mucsi, I.; Nacsa, J.; Hever, A.; Gyemánt, N.; Ugocsai, K.; Hegye, P.; Kiessig, S.T.; Gaal, D.; Lage, H.; et al. New silicon compounds as resistance modifiers against multidrug-resistant cancer cells. Anticancer Res. 2004, 24, 865–872. [Google Scholar] [PubMed]
- Kars, M.D.; Iseri, O.D.; Gunduz, U.; Ural, A.U.; Arpaci, F.; Molnar, J. Development of rational in vitro models for drug resistance in breast cancer and modulation of MDR by selected compounds. Anticancer Res. 2006, 26, 4559–4568. [Google Scholar]
- Molnar, J.; Ocsovszki, I.; Pusztai, R. Amyloid-beta interactions with ABC transporters and resistance modifiers. Anticancer Res. 2018, 38, 3407–3410. [Google Scholar] [CrossRef]
- Zalatnai, A.; Molnar, J. Effect of SILA-409, a new organosilicon multidrug- resistance modifier, on human pancreatic cancer xenografts. In Vivo 2006, 20, 137–140. [Google Scholar]
- Olszewski, U.; Zeillinger, R.; Kars, M.D.; Zalatnai, A.; Molnar, J.; Hamilton, G. Anticancer effects of the organosilicon multidrug-resistance modulator SILA 421. Anticancer Agents Med. Chem. 2012, 12, 663–671. [Google Scholar] [CrossRef]
- Martins, M.; Viveiros, M.; Ramos, J.; Couto, I.; Molnar, J.; Boeree, M.; Amaral, L. SILA-421, an inhibitor of efflux pumps of cancer cells, enhances the killing of intracellular extensively drug-resistant tuberculosis (XDR-TB). Int. J. Antimicrob. Ag. 2009, 33, 479–482. [Google Scholar] [CrossRef]
- Simons, S.O.; Kristiansen, J.E.; Hajos, G.; van der Laan, T.; Molnár, J.; Boeree, M.J.; van Ingen, J.; Christensen, J.B.; Viveiros, M.; Riedl, Z.; et al. Activity of the efflux pump inhibitor SILA 421 against drug-resistant tuberculosis. Int. J. Antimicrob. Ag. 2013, 41, 488–489. [Google Scholar] [CrossRef] [Green Version]
- De Knegt, G.J.; Bakker-Woudenberg, I.A.; van Soolingen, D.; Aarnoutse, R.; Boeree, M.J.; de Steenwinkel, J.E. SILA-421 activity in vitro against rifampicin-susceptible and rifampicin-resistant Mycobacterium tuberculosis, and in vivo in a murine tuberculosis model. Int. J. Antimicrob. Ag. 2015, 46, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Schelz, Z.; Martins, M.; Martins, A.; Viveiros, M.; Molnar, J.; Amaral, L. Elimination of plasmids by SILA compounds that inhibit efflux pumps of bacteria and cancer cells. In Vivo 2007, 21, 635–639. [Google Scholar]
- Tokuda, H.; Maoka, T.; Suzuiki, N.; Hohmann, J.; Vasas, A.; Engi, H.; Mucsi, I.; Olszewski, U.; Hamilton, G.; Amaral, L.; et al. Effects of two disiloxanes ALIS-409 and ALIS-421 on chemoprevention in model experiments. Anticancer Res. 2013, 33, 2021–2027. [Google Scholar] [PubMed]
- Wesołowska, O.; Wiśniewski, J.; Środa, K.; Krawczenko, A.; Bielawska-Pohl, A.; Paprocka, M.; Duś, D.; Michalak, K. 8-Prenylnaringenin is an inhibitor of multidrug resistance associated transporters, P-glycoprotein and MRP1. Eur. J. Pharmacol. 2010, 644, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Dobhal, M.P.; Li, G.; Gryshuk, A.; Graham, A.; Bhatanager, A.K.; Khaja, S.D.; Joshi, Y.C.; Sharma, M.C.; Oseroff, A.; Pandey, R.K. Structural modifications of plumieride isolated from Plumeria bicolor and the effect of these modifications on in vitro anticancer activity. J. Org. Chem. 2004, 69, 6165–6172. [Google Scholar] [CrossRef] [PubMed]
- Carella, A.; Roviello, V.; Iannitti, R.; Palumbo, R.; La Manna, S.; Marasco, D.; Trifuoggi, M.; Diana, R.; Roviello, G.N. Evaluating the biological properties of synthetic 4-nitrophenyl functionalized benzofuran derivatives with telomeric DNA binding and antiproliferative activities. Int. J. Biol. Macromol. 2019, 121, 77–88. [Google Scholar] [CrossRef]
- Środa-Pomianek, K.; Michalak, K.; Świątek, P.; Poła, A.; Palko-Łabuz, A.; Wesołowska, O. Increased lipid peroxidation, apoptosis and selective cytotoxicity in colon cancer cell line LoVo and its doxorubicin-resistant subline LoVo/Dx in the presence of newly synthesized phenothiazine derivatives. Biomed. Pharmacother. 2018, 106, 624–636. [Google Scholar] [CrossRef]
- Deo, K.M.; Sakoff, J.; Gilbert, J.; Zhang, Y.; Aldrich Wright, J.R. Synthesis, characterisation and influence of lipophilicity on cellular accumulation and cytotoxicity of unconventional platinum(iv) prodrugs as potent anticancer agents. Dalton Trans. 2019, 48, 17228–17240. [Google Scholar] [CrossRef]
- Środa-Pomianek, K.; Michalak, K.; Palko-Łabuz, A.; Poła, A.; Dzięgiel, P.; Puła, B.; Świątek, P.; Wesołowska, O. Cytotoxic and multidrug resistance reversal activity of phenothiazine derivative is strongly enhanced by theobromine, a phytochemical from cocoa. Eur. J. Pharmacol. 2019, 849, 124–134. [Google Scholar] [CrossRef]
- Grandi, M.; Geroni, C.; Giuliani, F.C. Isolation and characterization of a human colon adenocarcinoma cell line resistant to doxorubicin. Br. J. Cancer 1986, 54, 515–518. [Google Scholar] [CrossRef] [Green Version]
- Pastan, I.; Gottesman, M.M.; Ueda, K.; Lovelace, E.; Rutherford, A.V.; Willingham, M.C. A retrovirus carrying an MDR1 cDNA confers multidrug resistance and polarized expression of P-glycoprotein in MDCK cells. Proc. Natl. Acad. Sci. USA 1988, 85, 4486–4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Palko-Łabuz, A.; Środa-Pomianek, K.; Uryga, A.; Kostrzewa-Susłow, E.; Michalak, K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed. Pharmacother. 2017, 88, 232–241. [Google Scholar] [CrossRef]
- Chou, T.C.; Martin, N. CompuSyn for Drug Combinations: PC Software and User’s Guide: A Computer Program for Quantitation of Synergism and Antagonism in Drug Combinations, and the Determination of IC50 and ED50 and LD50 Values; ComboSyn Inc.: Paramus, NJ, USA, 2005. [Google Scholar]
- Riganti, C.; Miraglia, E.; Viarisio, D.; Costamagna, C.; Pescarmona, G. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005, 65, 516–525. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |






| Concentration (µM) | Ratio | Combination Index | |
|---|---|---|---|
| Dox | SILA-409 | ||
| 8.62 | 0.5 | 17.24:1 | 0.7772 |
| 17.24 | 0.5 | 34.48:1 | 0.6136 |
| SILA-421 | |||
| 8.62 | 1 | 8.62:1 | 0.6467 |
| 17.24 | 1 | 17.24:1 | 0.5632 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wesołowska, O.; Michalak, K.; Błaszczyk, M.; Molnár, J.; Środa-Pomianek, K. Organosilicon Compounds, SILA-409 and SILA-421, as Doxorubicin Resistance-Reversing Agents in Human Colon Cancer Cells. Molecules 2020, 25, 1654. https://doi.org/10.3390/molecules25071654
Wesołowska O, Michalak K, Błaszczyk M, Molnár J, Środa-Pomianek K. Organosilicon Compounds, SILA-409 and SILA-421, as Doxorubicin Resistance-Reversing Agents in Human Colon Cancer Cells. Molecules. 2020; 25(7):1654. https://doi.org/10.3390/molecules25071654
Chicago/Turabian StyleWesołowska, Olga, Krystyna Michalak, Maria Błaszczyk, Joseph Molnár, and Kamila Środa-Pomianek. 2020. "Organosilicon Compounds, SILA-409 and SILA-421, as Doxorubicin Resistance-Reversing Agents in Human Colon Cancer Cells" Molecules 25, no. 7: 1654. https://doi.org/10.3390/molecules25071654