Abstract
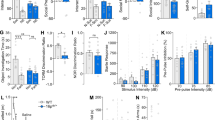
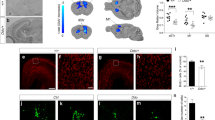
Methyl-CpG binding protein 2 (MeCP2) is a basic nuclear protein involved in the regulation of gene expression and microRNA processing. Duplication of MECP2-containing genomic segments causes MECP2 duplication syndrome, a severe neurodevelopmental disorder characterized by intellectual disability, motor dysfunction, heightened anxiety, epilepsy, autistic phenotypes, and early death. Reversal of the abnormal phenotypes in adult mice with MECP2 duplication (MECP2-TG) by normalizing the MeCP2 levels across the whole brain has been demonstrated. However, whether different brain areas or neural circuits contribute to different aspects of the behavioral deficits is still unknown. Here, we found that MECP2-TG mice showed a significant social recognition deficit, and were prone to display aversive-like behaviors, including heightened anxiety-like behaviors and a fear generalization phenotype. In addition, reduced locomotor activity was observed in MECP2-TG mice. However, appetitive behaviors and learning and memory were comparable in MECP2-TG and wild-type mice. Functional magnetic resonance imaging illustrated that the differences between MECP2-TG and wild-type mice were mainly concentrated in brain areas regulating emotion and social behaviors. We used the CRISPR-Cas9 method to restore normal MeCP2 levels in the medial prefrontal cortex (mPFC) and bed nuclei of the stria terminalis (BST) of adult MECP2-TG mice, and found that normalization of MeCP2 levels in the mPFC but not in the BST reversed the social recognition deficit. These data indicate that the mPFC is responsible for the social recognition deficit in the transgenic mice, and provide new insight into potential therapies for MECP2 duplication syndrome.






Similar content being viewed by others
References
Nan XS, Campoy FJ, Bird A. MeCP2 is a transcriptional repressor with abundant binding sites in genomic chromatin. Cell 1997, 88: 471–481.
Chahrour M, Jung SY, Shaw C, Zhou XB, Wong STC, Qin J, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320: 1224–1229.
Young JI, Hong EP, Castle JC, Crespo-Barreto J, Bowman AB, Rose MF, et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci U S A 2005, 102: 17551–17558.
Cheng TL, Wang ZZ, Liao QM, Zhu Y, Zhou WH, Xu WQ, et al. MeCP2 suppresses nuclear microRNA processing and dendritic growth by regulating the DGCR8/Drosha complex. Devel Cell 2014, 28: 547–560.
Cheng TL, Qiu ZL. MeCP2: multifaceted roles in gene regulation and neural development. Neurosci Bull 2014, 30: 601–609.
Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 1999, 23: 185–188.
Chahrour M, Zoghbi HY. The story of Rett syndrome: From clinic to neurobiology. Neuron 2007, 56: 422–437.
Van Esch H, Bauters M, Ignatius J, Jansen M, Raynaud M, Hollanders K, et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet 2005, 77: 442–453.
Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CMB, Schaaf CP, et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MECP2 duplication syndrome. Ann Neurol 2009, 66: 771–782.
Ramocki MB, Tavyev YJ, Peters SU. The MECP2 duplication syndrome. Am J Med Genet Part A 2010, 152A: 1079–1088.
Collins AL, Levenson JM, Vilaythong AP, Richman R, Armstrong DL, Noebels JL, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet 2004, 13: 2679–2689.
Samaco RC, Mandel-Brehm C, McGraw CM, Shaw CA, McGill BE, Zoghbi HY. Crh and Oprm1 mediate anxiety-related behavior and social approach in a mouse model of MECP2 duplication syndrome. Nat Genet 2012, 44: 206–211.
del Gaudio D, Fang P, Scaglia F, Ward PA, Craigen WJ, Glaze DG, et al. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet Med 2006, 8: 784–792.
Heckman LD, Chahrour MH, Zoghbi HY. Rett-causing mutations reveal two domains critical for MeCP2 function and for toxicity in MECP2 duplication syndrome mice. Elife 2014, 3.
Jiang MH, Ash RT, Baker SA, Suter B, Ferguson A, Park J, et al. Dendritic arborization and spine dynamics are abnormal in the mouse model of MECP2 duplication syndrome. J Neurosci 2013, 33: 19518–19533.
Nageshappa S, Carromeu C, Trujillo CA, Mesci P, Espuny-Camacho I, Pasciuto E, et al. Altered neuronal network and rescue in a human MECP2 duplication model. Molecular Psychiatry 2016, 21: 178–188.
Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron 2007, 56: 58–65.
Lu H, Ash RT, He LJ, Kee SE, Wang W, Yu DH, et al. Loss and gain of MeCP2 cause similar hippocampal circuit dysfunction that is rescued by deep brain stimulation in a Rett syndrome mouse model. Neuron 2016, 91: 739–747.
Sztainberg Y, Chen HM, Swann JW, Hao S, Tang B, Wu ZY, et al. Reversal of phenotypes in MECP2 duplication mice using genetic rescue or antisense oligos. Nature 2015, 528: 123–126.
Cook MN, Williams RW, Flaherty L. Anxiety-related behaviors in the elevated zero-maze are affected by genetic factors and retinal degeneration. Behav Neurosci 2001, 115: 468–476.
Cao W, Lin S, Xia QQ, Du YL, Yang Q, Zhang MY, et al. Gamma oscillation dysfunction in mPFC leads to social deficits in Neuroligin 3 R451C knockin mice. Neuron 2018, 97: 1253–1260.
Li RP, Liu XP, Sidabras JW, Paulson ES, Jesmanowicz A, Nencka AS, et al. Restoring susceptibility induced MRI signal loss in rat brain at 9.4 T: a step towards whole brain functional connectivity imaging. PLoS One 2015, 10.
Dorr AE, Lerch JP, Spring S, Kabani N, Henkelman RM. High resolution three-dimensional brain atlas using an average magnetic resonance image of 40 adult C57Bl/6 J mice. Neuroimage 2008, 42: 60–69.
Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011, 54: 2033–2044.
Zou QH, Zhu CZ, Yang YH, Zuo XN, Long XY, Cao QJ, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods 2008, 172: 137–141.
Fyffe SL, Neul JL, Samaco RC, Chao HT, Ben-Shachar S, Moretti P, et al. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron 2008, 59: 947–958.
Ito-Ishida A, Ure K, Chen H, Swann JW, Zoghbi HY. Loss of MeCP2 in parvalbumin-and somatostatin-expressing neurons in mice leads to distinct rett syndrome-like phenotypes. Neuron 2015, 88: 651–658.
Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci U S A 2009, 106: 21966–21971.
Finlay JM, Dunham GA, Isherwood AM, Newton CJ, Nguyen TV, Reppar PC, et al. Effects of prefrontal cortex and hippocampal NMDA NR1-subunit deletion on complex cognitive and social behaviors. Brain Res 2015, 1600: 70–83.
Liang B, Zhang LF, Barbera G, Fang WT, Zhang J, Chen XC, et al. Distinct and dynamic ON and OFF neural ensembles in the prefrontal cortex code social exploration. Neuron 2018, 100: 700–714.e9.
Niu B, Liu PP, Shen MJ, Liu C, Wang L, Wang FF, et al. GRK5 regulates social behavior via suppression of mTORC1 signaling in medial prefrontal cortex. Cereb Cortex 2018, 28: 421–432.
Tan Y, Singhal SM, Harden SW, Cahill KM, Nguyen DTM, Colon-Perez LM, et al. Oxytocin receptors are expressed by glutamatergic prefrontal cortical neurons that selectively modulate social recognition. J Neurosci 2019, 39: 3249–3263.
Swiech L, Heidenreich M, Banerjee A, Habib N, Li YQ, Trombetta J, et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat Biotechnol 2015, 33: U102–U286.
Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci 2014, 8.
Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci 2015, 16: 317–331.
Lohani S, Martig AK, Deisseroth K, Witten IB, Moghaddam B. Dopamine modulation of prefrontal cortex activity is manifold and operates at multiple temporal and spatial scales. Cell Rep 2019, 27: 99–114.e6.
Phillips ML, Robinson HA, Pozzo-Miller L. Ventral hippocampal projections to the medial prefrontal cortex regulate social memory. Elife 2019, 8.
Li Y, Missig G, Finger BC, Landino SM, Alexander AJ, Mokler EL, et al. Maternal and early postnatal immune activation produce dissociable effects on neurotransmission in mPFC-amygdala circuits. J Neurosci 2018, 38: 3358–3372.
Huang WC, Chen YJ, Page DT. Hyperconnectivity of prefrontal cortex to amygdala projections in a mouse model of macrocephaly/autism syndrome. Nat Commun 2016, 7.
Adachi M, Autry AE, Covington HE, Monteggia LM. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of rett syndrome. J Neurosci 2009, 29: 4218–4227.
Acknowledgements
We thank Wen-jing Chen and Kai-Wei Zhang, Institute of Neuroscience, Chinese Academy of Sciences, for assistance with fMRI data collection and advice on the manuscript. And we thank Zhi-Jiang Zhang and Sen Jin, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, for help with the rabies virus tracing experiments. This work was supported by National Natural Science Foundation of China grants (31625013 and 91732302); a Shanghai Brain-Intelligence Project of the Science and Technology Commission of Shanghai Municipality (16JC1420501); and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDBS01060200); Program of Shanghai Academic Research Leader, the Open Large Infrastructure Research of Chinese Academy of Sciences, and the Shanghai Municipal Science and Technology Major Project (2018SHZDZX05); and National Natural Science Foundation of China (81801354).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, B., Yuan, B., Dai, JK. et al. Reversal of Social Recognition Deficit in Adult Mice with MECP2 Duplication via Normalization of MeCP2 in the Medial Prefrontal Cortex. Neurosci. Bull. 36, 570–584 (2020). https://doi.org/10.1007/s12264-020-00467-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-020-00467-w