Abstract
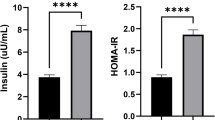
Bac Coronary artery disease (CAD) is the leading cause of death worldwide and most commonly develops as a result of atherosclerosis. ANGPTL8 is a secreted adipokine that regulates lipid metabolism and is associated with cardiometabolic diseases, including type 2 diabetes and CAD. However, the association between circulating ANGPTL8 levels and CAD is inconsistent among studies and the mechanism by which ANGPTL8 contributes to CAD development remains poorly understood. Here we sought to evaluate the relationship between ANGPTL8 levels and endothelial dysfunction and adipose tissue inflammation in CAD patients. Concentrations of ANGPTL8, adiponectin, TNF-α, IL6, hsCRP, ICAM-1, and VCAM-1 were measured by ELISA in serum samples from 192 CAD patients diagnosed with stenosis > 50% in at least one coronary artery by angiography and 71 individuals with normal heart function. Serum ANGPTL8 levels were significantly higher in CAD patients compared to controls (83.84 ± 23.25 ng/mL vs. 50.45 ± 17.73; p < 0.001), independent of adjustment for age, sex, BMI, smoking and statin use. ANGPTL8 could also differentiate CAD patients from controls with 82.3% specificity and 81.4% sensitivity (p < 0.001). Adiponectin levels were lower in CAD patients, while ICAM-1, VCAM-1, TNF-α, IL6, and hsCRP levels were higher compared to non-CAD controls (all p < 0.001). ANGPTL8 levels were associated with BMI in controls and with BMI, TG, and ICAM-1 in CAD patients. The presence of elevated ANGPTL8 levels in CAD patients and independent association with TG and ICAM-1 suggest a possible role related to endothelial dysfunction in the pathogenesis of atherosclerosis.




Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD (2011) Community prevalence of ideal cardiovascular health, by the American heart association definition, and relationship with cardiovascular disease incidence. J Am College Cardiol 57(16):1690–1696. https://doi.org/10.1016/j.jacc.2010.11.041
Bergheanu SC, Bodde MC, Jukema JW (2017) Pathophysiology and treatment of atherosclerosis : current view and future perspective on lipoprotein modification treatment. Neth Heart J 25(4):231–242. https://doi.org/10.1007/s12471-017-0959-2
Choi KM (2016) The impact of organokines on insulin resistance, inflammation, and atherosclerosis. Endocrinol Metabol 31(1):1–6. https://doi.org/10.3803/EnM.2016.31.1.1
Quagliarini F, Wang Y, Kozlitina J, Grishin NV, Hyde R, Boerwinkle E, Valenzuela DM, Murphy AJ, Cohen JC, Hobbs HH (2012) Atypical angiopoietin-like protein that regulates ANGPTL3. Proc Natl Acad Sci USA 109(48):19751–19756. https://doi.org/10.1073/pnas.1217552109
Ren G, Kim JY, Smas CM (2012) Identification of RIFL, a novel adipocyte-enriched insulin target gene with a role in lipid metabolism. Am J Physiol Endocrinol Metab 303(3):E334–351. https://doi.org/10.1152/ajpendo.00084.2012
Zhang R (2012) Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem Biophys Res Commun 424(4):786–792. https://doi.org/10.1016/j.bbrc.2012.07.038
Chi X, Britt EC, Shows HW, Hjelmaas AJ, Shetty SK, Cushing EM, Li W, Dou A, Zhang R, Davies BSJ (2017) ANGPTL8 promotes the ability of ANGPTL3 to bind and inhibit lipoprotein lipase. Mol Metab 6(10):1137–1149. https://doi.org/10.1016/j.molmet.2017.06.014
Haller JF, Mintah IJ, Shihanian LM, Stevis P, Buckler D, Alexa-Braun CA, Kleiner S, Banfi S, Cohen JC, Hobbs HH, Yancopoulos GD, Murphy AJ, Gusarova V, Gromada J (2017) ANGPTL8 requires ANGPTL3 to inhibit lipoprotein lipase and plasma triglyceride clearance. J Lipid Res 58(6):1166–1173. https://doi.org/10.1194/jlr.M075689
Lee SH, Rhee M, Kwon HS, Park YM, Yoon KH (2018) Serum betatrophin concentrations and the risk of incident diabetes: a nested case-control study from chungju metabolic disease cohort. Diabetes Metab J 42(1):53–62. https://doi.org/10.4093/dmj.2018.42.1.53
Hu H, Sun W, Yu S, Hong X, Qian W, Tang B, Wang D, Yang L, Wang J, Mao C, Zhou L, Yuan G (2014) Increased circulating levels of betatrophin in newly diagnosed type 2 diabetic patients. Diabetes Care 37(10):2718–2722. https://doi.org/10.2337/dc14-0602
Hu W, Shao X, Guo D, Hao H, Zhang Y, Xia M, Gong Y, Zhou H, Fan Y, Yu W (2017) Relationship of serum betatrophin with nonalcoholic fatty liver in a chinese population. PLoS ONE 12(1):e0170758. https://doi.org/10.1371/journal.pone.0170758
Abu-Farha M, Abubaker J, Al-Khairi I, Cherian P, Noronha F, Hu FB, Behbehani K, Elkum N (2015) Higher plasma betatrophin/ANGPTL8 level in Type 2 Diabetes subjects does not correlate with blood glucose or insulin resistance. Sci Rep 5:10949. https://doi.org/10.1038/srep10949
Abu-Farha M, Al-Khairi I, Cherian P, Chandy B, Sriraman D, Alhubail A, Al-Refaei F, AlTerki A, Abubaker J (2016) Increased ANGPTL3, 4 and ANGPTL8/betatrophin expression levels in obesity and T2D. Lipids Health Dis 15(1):181. https://doi.org/10.1186/s12944-016-0337-x
Al-Daghri NM, Rahman S, Sabico S, Amer OE, Wani K, Ansari MG, Al-Attas OS, Kumar S, Alokail MS (2016) Circulating betatrophin in healthy control and type 2 diabetic subjects and its association with metabolic parameters. J Diabetes Complications 30(7):1321–1325. https://doi.org/10.1016/j.jdiacomp.2016.05.023
Al-Rawashdeh A, Kasabri V, Bulatova N, Akour A, Zayed A, Momani M, Khawaja N, Bustanji H, Hyasat D (2017) The correlation between plasma levels of oxytocin and betatrophin in non-diabetic and diabetic metabolic syndrome patients: a cross sectional study from Jordan. Diabetes Metab Syndr 11(1):59–67. https://doi.org/10.1016/j.dsx.2016.08.008
Chen X, Lu P, He W, Zhang J, Liu L, Yang Y, Liu Z, Xie J, Shao S, Du T, Su X, Zhou X, Hu S, Yuan G, Zhang M, Zhang H, Liu L, Wang D, Yu X (2015) Circulating betatrophin levels are increased in patients with type 2 diabetes and associated with insulin resistance. J Clini Endocrinol Metab 100(1):E96–100. https://doi.org/10.1210/jc.2014-2300
Ebert T, Kralisch S, Hoffmann A, Bachmann A, Lossner U, Kratzsch J, Bluher M, Stumvoll M, Tonjes A, Fasshauer M (2014) Circulating angiopoietin-like protein 8 is independently associated with fasting plasma glucose and type 2 diabetes mellitus. J Clini Endocrinol Metab 99(12):E2510–2517. https://doi.org/10.1210/jc.2013-4349
Ejarque M, Borlaug M, Vilarrasa N, Martinez-Perez B, Llaurado G, Megia A, Helland T, Gutierrez C, Serena C, Folkestad O, Nunez-Roa C, Roche K, Casajoana A, Fradera R, Gonzalez-Clemente JM, Lopez M, Mohn AC, Nedrebo BG, Nogueiras R, Mellgren G, Ferno J, Fernandez-Veledo S, Vendrell J (2017) Angiopoietin-like protein 8/betatrophin as a new determinant of type 2 diabetes remission after bariatric surgery. Transl Res 184(35–44):e34. https://doi.org/10.1016/j.trsl.2017.03.001
Espes D, Martinell M, Carlsson PO (2014) Increased circulating betatrophin concentrations in patients with type 2 diabetes. Int J Endocrinol 2014:323407. https://doi.org/10.1155/2014/323407
Fu Z, Berhane F, Fite A, Seyoum B, Abou-Samra AB, Zhang R (2014) Elevated circulating lipasin/betatrophin in human type 2 diabetes and obesity. Sci Rep 4:5013. https://doi.org/10.1038/srep05013
Huang Y, Fang C, Guo H, Hu J (2016) Increased angiopoietin-like protein 8 levels in patients with type 2 diabetes and cardiovascular disease. Diabetes Res Clin Pract 120:229–231. https://doi.org/10.1016/j.diabres.2016.08.017
Li S, Liu D, Li L, Li Y, Li Q, An Z, Sun X, Tian H (2016) Circulating betatrophin in patients with type 2 diabetes: a meta-analysis. J Diabetes Res 2016:6194750. https://doi.org/10.1155/2016/6194750
Luo M, Zhang Z, Peng Y, Wang S, Peng D (2018) The negative effect of ANGPTL8 on HDL-mediated cholesterol efflux capacity. Cardiovasc Diabetol 17(1):142. https://doi.org/10.1186/s12933-018-0785-x
Yamada H, Saito T, Aoki A, Asano T, Yoshida M, Ikoma A, Kusaka I, Toyoshima H, Kakei M, Ishikawa SE (2015) Circulating betatrophin is elevated in patients with type 1 and type 2 diabetes. Endocr J 62(5):417–421. https://doi.org/10.1507/endocrj.EJ14-0525
Yang L, Song J, Zhang X, Xiao L, Hu X, Pan H, Qin L, Liu H, Ge B, Zheng T (2018) Association of Serum Angiopoietin-Like Protein 8 With Albuminuria in Type 2 Diabetic Patients: Results From the GDMD Study in China. Front Endocrinol 9:414. https://doi.org/10.3389/fendo.2018.00414
Yin Y, Ding X, Peng L, Hou Y, Ling Y, Gu M, Wang Y, Peng Y, Sun H (2017) Increased serum angptl8 concentrations in patients with prediabetes and type 2 diabetes. J Diabetes Res 2017:8293207. https://doi.org/10.1155/2017/8293207
Yue S, Wu J, Zhang J, Liu L, Chen L (2016) The relationship between betatrophin levels in blood and t2dm: a systematic review and meta-analysis. Dis Markers 2016:9391837. https://doi.org/10.1155/2016/9391837
Zheng T, Ge B, Liu H, Chen B, Qin L, Xiao L, Song J (2018) Triglyceride-mediated influence of serum angiopoietin-like protein 8 on subclinical atherosclerosis in type 2 diabetic patients: results from the GDMD study in China. Cardiovasc Diabetol 17(1):84. https://doi.org/10.1186/s12933-018-0687-y
Hong BS, Liu J, Zheng J, Ke W, Huang Z, Wan X, He X, Xiao H, Li Y (2018) Angiopoietin-like protein 8/betatrophin correlates with hepatocellular lipid content independent of insulin resistance in non-alcoholic fatty liver disease patients. J Diabetes Investig 9(4):952–958. https://doi.org/10.1111/jdi.12792
Lee Y-h, Lee S-G, Lee CJ, Kim SH, Song Y-M, Yoon MR, Jeon BH, Lee JH, Lee B-W, Kang ES, Lee HC, Cha B-S (2016) Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: animal and human studies. Scientific Rep 6:24013. https://doi.org/10.1038/srep24013
Abu-Farha M, Cherian P, Qaddoumi MG, AlKhairi I, Sriraman D, Alanbaei M, Abubaker J (2018) Increased plasma and adipose tissue levels of ANGPTL8/Betatrophin and ANGPTL4 in people with hypertension. Lipids Health Dis 17(1):35. https://doi.org/10.1186/s12944-018-0681-0
Abu-Farha M, Abubaker J, Al-Khairi I, Cherian P, Noronha F, Kavalakatt S, Khadir A, Behbehani K, Alarouj M, Bennakhi A, Elkum N (2016) Circulating angiopoietin-like protein 8 (betatrophin) association with HsCRP and metabolic syndrome. Cardiovasc Diabetol 15:25. https://doi.org/10.1186/s12933-016-0346-0
Jiao X, He J, Yang Y, Yang S, Li J, Qin Y (2018) Associations between circulating full-length angiopoietin-like protein 8 levels and severity of coronary artery disease in Chinese non-diabetic patients: a case-control study. Cardiovasc diabetol 17(1):92. https://doi.org/10.1186/s12933-018-0736-6
Niki H, Kishimoto Y, Ibe S, Saita E, Sasaki K, Miura K, Ikegami Y, Ohmori R, Kondo K, Momiyama Y (2019) Associations between plasma betatrophin levels and coronary and peripheral artery disease. J Atheroscler Thromb 26(6):573–581. https://doi.org/10.5551/jat.46508
Qin YWY, Wang Y, Tang C, Du J (2015) increased circulating betatrophin concentrations in patients with coronary heart disease. Arterioscler Thromb Vasc Biol 35:A336
Luo M, Su X, Yi Y, Yang Y, Peng D (2018) Apolipoprotein CIII may mediate the impacts of angiopoietin-like protein 8 on triglyceride metabolism. Lipids Health Dis 17(1):160. https://doi.org/10.1186/s12944-018-0777-6
Leiherer AMA, Geiger K, Saely CH, Brandtner E-M, Ebner J, Larcher B, Mader A, Fraunberger P, Drexel H (2018) Betatrophin predicts cardiovascular events independently from the presence of type 2 diabetes and coronary artery disease. Diabetes 287:67
Fu Z, Abou-Samra AB, Zhang R (2014) An explanation for recent discrepancies in levels of human circulating betatrophin. Diabetologia 57(10):2232–2234. https://doi.org/10.1007/s00125-014-3346-1
Fischer JJ, Samady H, McPherson JA, Sarembock IJ, Powers ER, Gimple LW, Ragosta M (2002) Comparison between visual assessment and quantitative angiography versus fractional flow reserve for native coronary narrowings of moderate severity. Am J Cardiol 90(3):210–215. https://doi.org/10.1016/s0002-9149(02)02456-6
Zhang R, Abou-Samra AB (2013) Emerging roles of Lipasin as a critical lipid regulator. Biochem Biophys Res Commun 432(3):401–405. https://doi.org/10.1016/j.bbrc.2013.01.129
Wang Y, Quagliarini F, Gusarova V, Gromada J, Valenzuela DM, Cohen JC, Hobbs HH (2013) Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc Natl Acad Sci USA 110(40):16109–16114. https://doi.org/10.1073/pnas.1315292110
Niki H, Kishimoto Y, Ibe S, Saita E, Sasaki K, Miura K, Ikegami Y, Ohmori R, Kondo K, Momiyama Y (2018) Associations between plasma betatrophin levels and coronary and peripheral artery disease. J Atherosclerosis Thrombosis advpub. https://doi.org/10.5551/jat.46508
Liu D, Li S, He H, Yu C, Li X, Liang L, Chen Y, Li J, Li J, Sun X, Tian H, An Z (2017) Increased circulating full-length betatrophin levels in drug-naïve metabolic syndrome. Oncotarget 8(11):17510–17517. https://doi.org/10.18632/oncotarget.15102
Christopoulou E, Elisaf M, Filippatos T (2019) Effects of angiopoietin-like 3 on triglyceride regulation, glucose homeostasis, and diabetes. Dis Markers 2019:6578327. https://doi.org/10.1155/2019/6578327
Vatner DF, Goedeke L, Camporez JG, Lyu K, Nasiri AR, Zhang D, Bhanot S, Murray SF, Still CD, Gerhard GS, Shulman GI, Samuel VT (2018) Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia 61(6):1435–1446. https://doi.org/10.1007/s00125-018-4579-1
Abu-Farha M, Al Madhoun A, Abubaker J (2016) The rise and the fall of betatrophin/angptl8 as an inducer of β-cell proliferation. J Diabetes Res 2016:4860595. https://doi.org/10.1155/2016/4860595
Tseng YH, Yeh YH, Chen WJ, Lin KH (2014) Emerging regulation and function of betatrophin. Int J Mol Sci 15(12):23640–23657. https://doi.org/10.3390/ijms151223640
Abu-Farha M, Sriraman D, Cherian P, AlKhairi I, Elkum N, Behbehani K, Abubaker J (2016) Circulating ANGPTL8/Betatrophin is increased in obesity and reduced after exercise training. PLoS ONE 11(1):e0147367. https://doi.org/10.1371/journal.pone.0147367
Ramos TN, Bullard DC, Barnum SR (2014) ICAM-1: isoforms and phenotypes. J Immunol 192(10):4469–4474. https://doi.org/10.4049/jimmunol.1400135
Carbone C, Piro G, Merz V, Simionato F, Santoro R, Zecchetto C, Tortora G, Melisi D (2018) Angiopoietin-like proteins in angiogenesis, inflammation and cancer. Int J Mol Sci 19(2):431–453. https://doi.org/10.3390/ijms19020431
Zhang Y, Guo X, Yan W, Chen Y, Ke M, Cheng C, Zhu X, Xue W, Zhou Q, Zheng L, Wang S, Wu B, Liu X, Ma L, Huang L, Huang K (2017) ANGPTL8 negatively regulates NF-κB activation by facilitating selective autophagic degradation of IKKγ. Nat Commun 8(1):2164. https://doi.org/10.1038/s41467-017-02355-w
Huang Y, Ye Z, Ma T, Li H, Zhao Y, Chen W, Wang Y, Yan X, Gao Y, Li Z (2018) Carbon monoxide (CO) modulates hydrogen peroxide (H2O2)-mediated cellular dysfunction by targeting mitochondria in rabbit lens epithelial cells. Exp Eye Res 169:68–78. https://doi.org/10.1016/j.exer.2018.01.023
Jiao Y, Mao X, Chang X, Abudureyimu K, Zhang C, Lu J, Wang Y, Nuermaimaiti N, Aisa Y, Gong X, Guan Y (2014) Adenovirus36 infection expresses cellular APMI and Visfatin genes in overweight Uygur individuals. Diagn Pathol 9:83. https://doi.org/10.1186/1746-1596-9-83
Acknowledgements
The authors wish to thank Brandon S. Davies, PhD from Department of Biochemistry, Fraternal Order of Eagles Diabetes Research Center, and Obesity Research and Education Initiative, University of Iowa Carver College of Medicine, Iowa City for helpful feedback and comments in preparation of the manuscript.
Funding
The study was funded by Molecular Medicine Research Center, Hamadan University of Medical Sciences (No. 9609075609).
Author information
Authors and Affiliations
Contributions
R.F., H.S., and N.Z. generated and developed the study hypothesis and design. R.F., H.S., N.M., F.E., and H.A. collected the data. R.F. analyzed and interpreted the data. R.F., H.S., J.K.D., N.M., M.M., F.E., H.A., and N.Z. drafted and revised the manuscript. R.F., J.K.D., M.M., and N.Z. completed the final version of manuscript. All authors read and confirmed the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study approved by the Ethics committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1396.535) and was conducted in accordance with the Declaration of Helsinki.
Informed consent
Informed written consent was obtained from all study participants.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fadaei, R., Shateri, H., DiStefano, J.K. et al. Higher circulating levels of ANGPTL8 are associated with body mass index, triglycerides, and endothelial dysfunction in patients with coronary artery disease. Mol Cell Biochem 469, 29–39 (2020). https://doi.org/10.1007/s11010-020-03725-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03725-7