Abstract
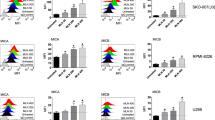
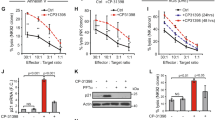
Immunomodulatory drugs (IMiDs) lenalidomide and pomalidomide show remarkable antitumor activity in multiple myeloma (MM) via directly inhibiting MM-cell growth in the bone marrow (BM) microenvironment and promoting immune effector cell function. They are known to bind to the ubiquitin 3 ligase CRBN complex and thereby triggering degradation of IKZF1/3. In this study, we demonstrate that IMiDs also directly bind and activate zeta-chain-associated protein kinase-70 (Zap-70) via its tyrosine residue phosphorylation in T cells. IMiDs also triggered phosphorylation of Zap-70 in natural killer (NK) cells. Importantly, increased granzyme-B (GZM-B) expression and NK-cell activity triggered by IMiDs is associated with Zap-70 activation and inhibited by Zap-70 knockdown (KD), independent of CRBN. We also demonstrate a second mechanism whereby IMiDs trigger GZM-B and NK cytotoxicity which is CRBN and IKZF3 mediated, and inhibited or enhanced by KD of CRBN or IKZF3, respectively, independent of Zap-70. Our studies therefore show that IMiDs can enhance NK and T-cell cytotoxicity in (1) ZAP-70-mediated CRBN independent, as well as (2) CRBN-mediated ZAP-70 independent mechanisms; and provide the framework for developing novel therapeutics to activate Zap-70 and thereby enhance T and NK anti-MM cytotoxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai Y-T, et al. Thalidomide and its analogues overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–50.
Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, et al. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–30.
Anderson KC. The rapid evolution of novel therapies in multiple myeloma. J Natl Compr Cancer Netw. 2016;14:493–96.
Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, et al. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327:1345–50.
Lopez-Girona A, Mendy D, Ito T, Miller K, Gandhi AK, Kang J, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. Leukemia. 2012;26:2326–35.
Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343:301–5.
Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science. 2014;343:305–9.
Hideshima T, Cottini F, Nozawa Y, Seo HS, Ohguchi H, Samur MK, et al. p53-related protein kinase confers poor prognosis and represents a novel therapeutic target in multiple myeloma. Blood. 2017;129:1308–19.
LeBlanc R, Hideshima T, Catley LP, Shringarpure R, Burger R, Mitsiades N, et al. Immunomodulatory drug costimulates T cells via the B7-CD28 pathway. Blood. 2004;103:1787–90.
Gorgun G, Calabrese E, Soydan E, Hideshima T, Perrone G, Bandi M, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010;116:3227–37.
Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4 (CRBN). Br J Haematol. 2014;164:811–21.
Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–16.
Hayashi T, Hideshima T, Akiyama M, Podar K, Yasui H, Raje N, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128:192–203.
Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108:618–21.
Reddy N, Hernandez-Ilizaliturri FJ, Deeb G, Roth M, Vaughn M, Knight J, et al. Immunomodulatory drugs stimulate natural killer-cell function, alter cytokine production by dendritic cells, and inhibit angiogenesis enhancing the anti-tumour activity of rituximab in vivo. Br J Haematol. 2008;140:36–45.
Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, et al. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin Cancer Res. 2008;14:4650–57.
Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, et al. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood. 2013;121:423–30.
Pittari G, Vago L, Festuccia M, Bonini C, Mudawi D, Giaccone L, et al. Restoring natural killer cell immunity against multiple myeloma in the era of new drugs. Front Immunol. 2017;8:1444.
Neuber B, Dai J, Waraich WA, Awwad MHS, Engelhardt M, Schmitt M, et al. Lenalidomide overcomes the immunosuppression of regulatory CD8(+)CD28(−) T-cells. Oncotarget. 2017;8:98200–14.
Wang H, Kadlecek TA, Au-Yeung BB, Goodfellow HE, Hsu LY, Freedman TS, et al. ZAP-70: an essential kinase in T-cell signaling. Cold Spring Harb Perspect Biol. 2010;2:a002279.
Hagner PR, Chiu H, Ortiz M, Apollonio B, Wang M, Couto S, et al. Activity of lenalidomide in mantle cell lymphoma can be explained by NK cell-mediated cytotoxicity. Br J Haematol. 2017;179:399–409.
van de Donk N. Immunomodulatory effects of CD38-targeting antibodies. Immunol Lett. 2018;199:16–22.
Leonard JP, Jung SH, Johnson J, Pitcher BN, Bartlett NL, Blum KA, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol. 2015;33:3635–40.
Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, et al. AUGMENT: a phase III study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019;37:1188–99.
Liu Y, Wang Y, Yang J, Bi Y, Wang H. ZAP-70 in chronic lymphocytic leukemia: a meta-analysis. Clin Chim Acta. 2018;483:82–8.
Kronke J, Fink EC, Hollenbach PW, MacBeth KJ, Hurst SN, Udeshi ND, et al. Lenalidomide induces ubiquitination and degradation of CK1alpha in del(5q) MDS. Nature. 2015;523:183–8.
Paolini R, Molfetta R, Piccoli M, Frati L, Santoni A. Ubiquitination and degradation of Syk and ZAP-70 protein tyrosine kinases in human NK cells upon CD16 engagement. Proc Natl Acad Sci USA. 2001;98:9611–6.
Yang M, Chen T, Li X, Yu Z, Tang S, Wang C, et al. K33-linked polyubiquitination of Zap70 by Nrdp1 controls CD8(+) T cell activation. Nat Immunol. 2015;16:1253–62.
Wargnier A, Legros-Maida S, Bosselut R, Bourge JF, Lafaurie C, Ghysdael CJ, et al. Identification of human granzyme B promoter regulatory elements interacting with activated T-cell-specific proteins: implication of Ikaros and CBF binding sites in promoter activation. Proc Natl Acad Sci USA. 1995;92:6930–4.
Ito T, Handa H. Recent topics in IMiDs and cereblon. Rinsho Ketsueki. 2017;58:2067–73.
Matyskiela ME, Zhang W, Man HW, Muller G, Khambatta G, Baculi F, et al. A cereblon modulator (CC-220) with improved degradation of Ikaros and Aiolos. J Med Chem. 2018;61:535–42.
Acknowledgements
We thank Taiho Pharmaceuticals for providing Pom-immobilized beads. This study was supported by the National Institute of Health Grant; SPORE-P50CA100707 (KCA), R01-CA050947 (KCA) and R01-CA178264 (TH and KCA); and the Sheldon and Miriam Medical Research Foundation (KCA). KCA is an American Cancer Society Clinical Research Professor.
Author information
Authors and Affiliations
Contributions
TH coordinated activities from all authors and performed Western blot, Zap-70 kinase assay, siRNA (Zap-70, CRBN, IKZF1, IKZF3) transfection, NK assay, as well as cell toxicity assay (MTT and cell count), and wrote the paper. DO performed real-time qPCR (GZM-B), NK assay, and purified T cells and NK cells from healthy volunteers. JL performed siRNA (Zap-70, CRBN, IKZF1, and IKZF3) transfection, TH performed Zap-70 pull-down by Pom-beads. KK performed real-time qPCR (GZM-B). JB analyzed the data. WM performed NMR and wrote the paper. KCA managed the project and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
KCA serves on advisory boards to Celgene, Millennium, Janssen, Sanofi, Bristol Myers Squibb, Gilead, Precision Biosciences, and Tolero, and is a Scientific Founder of OncoPep and C4 Therapeutics. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the paper apart from those disclosed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Hideshima, T., Ogiya, D., Liu, J. et al. Immunomodulatory drugs activate NK cells via both Zap-70 and cereblon-dependent pathways. Leukemia 35, 177–188 (2021). https://doi.org/10.1038/s41375-020-0809-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-020-0809-x
This article is cited by
-
AQP9 and ZAP70 as immune-related prognostic biomarkers suppress proliferation, migration and invasion of laryngeal cancer cells
BMC Cancer (2022)
-
Harnessing natural killer cells for cancer immunotherapy: dispatching the first responders
Nature Reviews Drug Discovery (2022)
-
Natural killer cell awakening: unleash cancer-immunity cycle against glioblastoma
Cell Death & Disease (2022)
-
Unleashing the power of NK cells in anticancer immunotherapy
Journal of Molecular Medicine (2022)