Abstract
The burden of chronic obstructive pulmonary disease (COPD) to patients and health services is steadily increasing. Self-management supported by mobile device applications could improve outcomes for people with COPD. Our aim was to synthesize evidence on the effectiveness of mobile health applications compared with usual care. A systematic review was conducted to identify randomized controlled trials. Outcomes of interest included exacerbations, physical function, and Quality of Life (QoL). Where possible, outcome data were pooled for meta-analyses. Of 1709 citations returned, 13 were eligible trials. Number of exacerbations, quality of life, physical function, dyspnea, physical activity, and self-efficacy were reported. Evidence for effectiveness was inconsistent between studies, and the pooled effect size for physical function and QoL was not significant. There was notable variation in outcome measures used across trials. Developing a standardized outcome-reporting framework for digital health interventions in COPD self-management may help standardize future research.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD) affects the functional capacity of the lungs, characterized by airflow limitation and is commonly progressive1. One in 20 adults aged over 40 years old in the United Kingdom have diagnosed COPD and it is projected to be the fourth leading cause of global mortality by 20302. Despite the preventable and treatable nature of the condition3, it poses a high financial burden to the healthcare systems globally. In England, the annual direct healthcare costs of COPD were estimated to be £1.5 billion in 2011, with severe exacerbations costing £3726 per event4. There are also substantial indirect and intangible costs associated with COPD, which are much harder to quantify, but include time lost from work, impact on family, and additional social and care costs5.
Acute exacerbations of COPD are defined as acute events leading to the worsening of respiratory condition beyond normal daily variation3. Increased frequency of exacerbations and ongoing, progressive development of the condition itself can significantly impact QoL and increase the risk of mortality6. Initial studies incorporating technology into self-management interventions for COPD patients combined phone calls with weekly visits from health professionals, and indicated that this strategy could result in fewer exacerbation-related hospital attendances7. Increasing attention to the potential for self-management has highlighted the role of digital health technologies. The capabilities of mobile device technologies have substantially increased, and applications can facilitate access to and awareness of self-management strategies for patients living with long-term conditions such as COPD.
Studies exploring patient experience and acceptability of apps have shown promise8, suggesting that such technology may be able to complement current clinical care. However, the evidence base to support this approach is currently unclear. Several systematic reviews have been conducted exploring applications to support self-management of COPD, but questions remain regarding their potential to improve clinical and nonclinical outcomes. Meta-analyses to date have pooled trials investigating hospital admissions9, physical activity10, physical function10, dyspnea10, and exacerbations11. However, reviews to date have used varying eligibility criteria for inclusion, excluding tablet computers11, excluding trials with any healthcare professional input12, excluding trials shorter than 1 month in duration9, or only including trials reporting hospitalization or exacerbation events9,11. With technologies rapidly evolving, it is also important to identify the effective and less effective components of current interventions to help inform future interventions, so this review will provide a detailed description of each intervention. The aim of this systematic review was to build on existing reviews by synthesizing and appraising evidence on the effectiveness of mobile applications (encompassing smartphones, tablet computers, and accompanying devices such as wearable sensors) in people with COPD.
Results
Study selection
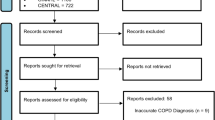
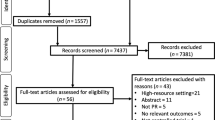
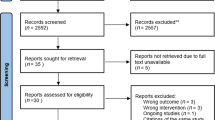
The initial search identified 1709 citations; 738 duplicates were removed. After screening titles and abstracts, 933 papers were excluded. Thirty-eight trials were assessed using full texts and 11 were deemed eligible for inclusion. After screening reference lists of the included trials, two additional trials were identified, resulting in a total of 13 trials for inclusion (Fig. 1).
Study characteristics
Study characteristics are reported in Table 1. All 13 trials13,14,15,16,17,18,19,20,21,22,23,24 were published after 2008, with most (12 of 13) published since 2011. Trials were conducted in a number of countries and settings; however, most were in the Netherlands17,18,19,20 or the United Kingdom13,16,22,23. Five trials14,17,18,23,25 included fewer than 50 participants and the largest number of participants was 34321. Across all 13 trials, the total number of participants was 1447. Participants were generally aged ≥60 years, and the proportion of males and females was similar within trials. One study25 included male participants only and another14 only included one female participant. Baseline measures of lung function were identified in nine trials13,14,15,16,17,18,20,21,25. Study duration varied from 2 weeks23 to 12 months15,16,20,22.
A description of the interventions is outlined in Supplementary Table 1. Eight of the interventions13,15,17,19,21,23,24,25 were smartphone-based, using custom applications whereby participants entered COPD symptom data and received custom or automated feedback based on their responses. Healthcare professional involvement through active monitoring of entered data, clinical advice, or intervention on deteriorating observations was noted in seven trials14,16,18,19,20,22,24. Eleven trials13,14,15,17,18,19,20,21,23,24,25 delivered the intervention through a smartphone and two16,22 utilized a mobile tablet device. Five trials14,18,21,22,23 provided participants with a monitoring device such as a pulse oximeter and a pedometer, which linked to the applications to provide additional data.
Risk of bias within studies
An overview of the results for the bias assessment is presented in Fig. 2. Random sequence generation was clearly carried out in 12 trials, with one trial unclear on random sequence generation15. Six trials14,15,19,20,24,25 were unclear on concealment of allocation. Risk of selective reporting was considered low in 12 trials with the remaining trial18 classified as having a high risk of bias. Regarding blinding of participants to intervention, four trials19,20,21,23 were considered at high risk of bias, eight trials14,15,16,17,18,22,24,25 did not provide sufficient information for assessment about the degree of participant blinding, and the remaining trial13 was considered at low risk. Halpin et al. (2011) was judged to be at low risk because both control and intervention participants had access to a smartphone application, with only the intervention group receiving alerts, and participants were not informed of their allocation13. Similarly, four trials14,18,23,24 were considered at high risk of bias for the blinding of outcome assessments, three trials15,18,25 were unclear, and the remaining six trials13,16,19,20,21,22,23 at low risk of bias.
Primary outcome
Five trials13,14,16,22,25 reported the frequency of COPD exacerbations that led to clinical intervention (hospitalization or managed in the community). However, only one of these trials14 reported pre-intervention and post-intervention exacerbation data. One trial16 presented patient self-reported exacerbations but only post-intervention data. A summary of the main findings of the included trials can be seen in Table 2.
Other outcomes
Physical function
Physical function was reported in five trials (Table 3)15,18,20,21,25. One trial25 recorded the incremental shuttle-walking test and showed the results that were neither statistically significant nor indicated a clinically important difference between intervention and control groups. The other trials15,18,20,21 used the 6-minute walk test. Only one trial21 recorded a significant difference between the groups in the post-intervention period. No difference between intervention and usual care was found for the 6-minute walk test (mean difference, 8.38 m, 95% CI, −4.40 to 21.17, p = 0.20; Fig. 3). The I2 estimate was 52% that represents moderate-to-substantial heterogeneity.
Quality of life (QoL)
Twelve of the 13 trials reported QoL; two of these trials15,21 reported two different quality-of-life measures. Across all 12 trials, 14 quality-of-life measures were reported (Table 4). Only one trial25 reported the SF-12 measure, reporting a significant difference between intervention and control post-intervention. Two trials15,19 used the SF-36 measure, but these did not identify statistically significant differences. One trial21 reported the individual mental, functional, and symptom domains of the Chronic COPD Questionnaire. There was a significant difference between the intervention and control groups in the Functional CCQ measure post intervention but not in other domains. Two trials17,18 recorded the total CCQ score, but the results were not significant. The Chronic Respiratory Disease Questionnaire was reported in full by one trial15, and partially by two trials14,20 (only reporting the emotion and mastery domains). These three trials reported non-significant results for these domains. Three trials13,16,22 reported the St. George’s Respiratory Questionnaire and two trials21,23 reported the COPD Assessment Test measure of QoL, but none of them showed significant differences between intervention and control groups. The 12 trials reporting QoL were assessed for inclusion for the meta-analysis, but trials that did not report a total or summative score were excluded, resulting in a total of eight eligible trials (Fig. 4). The trial by Nguyen et al. (2013) reported two total scores reflecting QoL (Chronic Respiratory Disease Questionnaire and SF-36); the disease-specific scale (Chronic Respiratory Disease Questionnaire) was included in the meta-analysis. No difference in QoL was found between mobile device application intervention and usual care (standardized mean difference, −0.4 points; 95% CI, −0.86 to 0.05, p = 0.08). The I2 estimate was 83% that represents considerable heterogeneity. The minimal clinically important differences for the back-translated standardized mean differences are presented in Supplementary Table 2.
Dyspnea
Five trials14,15,17,20,24 reported data relating to dyspnea (Supplementary Table 3). Three trials14,15,20 used the dyspnea component of the Chronic Respiratory Disease Questionnaire measure, while the other two trials17,24 used the modified Medical Research Council dyspnea scale. Only one trial24 reported a statistically significant difference between groups.
Fatigue
Five trials14,17,18,20,23 reported data concerning fatigue (Supplementary Table 4). Two trials14,20 reported the fatigue component of the CRQ, two trials17,18 reported the Multidimensional Fatigue Inventory, and one trial23 used the Functional Assessment of Chronic Illness Therapy measure. None of these trials reported significant improvements in the intervention arm compared with control.
Physical activity
Five trials17,18,19,20,21 reported device-based levels of physical activity (Supplementary Table 5). Four trials recorded physical activity using accelerometers, while the remaining trial used pedometers. Only one trial19 reported a statistically significant difference in physical activity outcomes between groups in the post-intervention period. Two of these five trials also provided self-reported levels of physical activity, using the Moderate Physical Activity questionnaire21 and the Baecke Physical Activity Questionnaire18. Both trials reported non-significant changes from baseline.
Self-efficacy
Four trials15,16,19,23 reported patient self-efficacy (Supplementary Table 6). The employed measures focused on dyspnea15, falls23, exercise19, and self-efficacy more generally16,19. No trials recorded statistically significant findings.
Anxiety and depression
Two trials16,23 reported anxiety and depression, using the Hospital Anxiety and Depression Scale (HADS), and no statistically significant differences were observed.
Discussion
This systematic review provided a comprehensive description and summarized the findings of mobile device application interventions for COPD self-management. The interventions identified were heterogeneous in nature, including the components (such as the inclusion of periphery devices), the degree and frequency of involvement of healthcare professionals, and frequency of participant-performed measurements and data entry. It remains unclear whether mobile device applications are more effective at preventing exacerbations when compared with usual care.
As only published trials were eligible for inclusion, there is potential for publication bias within the review. Also, the risk assessment bias tool was challenging to implement because blinding of participants in digital health interventions where the comparator is usual care may not be feasible to implement. In addition, our ability to pool further outcome measures using meta-analysis was limited, given the variety of outcome measures used across the trials. There are also limitations to interpreting summary estimates from pooled data, particularly when the design of the studies, scales used to assess effectiveness, and interventions tested are heterogeneous and use varying follow-up durations. However, the present review was prospectively registered on a database of systematic reviews and included trials published in any language in several databases from inception. A sensitive search strategy was developed, and screening of citations was performed independently, minimizing the risk of bias at review level. The review was inclusive of a broad range of outcome measures, contributing to its comprehensive nature.
Although exacerbations can negatively impact QoL26 and increase mortality27, only five of the included trials reported exacerbations. Only one of these trials reported pre-intervention and post-intervention exacerbation frequency14, and exacerbations were reported using a wide range of metrics, including those exacerbations managed in the community and leading to hospitalization. An 80% reduction in likelihood of having an exacerbation has been demonstrated previously in a meta-analysis comparing a smartphone intervention with usual care11. However, the meta-analysis showed moderate heterogeneity in this healthcare professional contact, in part possible because of the small sample size of the three trials pooled. It is unclear if reporting the number of contacts with healthcare professionals is a suitable outcome measure to represent COPD exacerbations; digital interventions can offer an alternate means of contacting a healthcare professional, impacting the accuracy of assessing exacerbation frequency in this way. With prevention and management of exacerbations being a key feature of COPD care, and an increasing interest in predicting the onset of exacerbations28,29,30, future trials are recommended to consider this when reporting exacerbations to more accurately quantify the impact of digital interventions on this important clinical outcome.
The trials identified in this systematic review do not yet provide strong evidence for implementing mobile digital health interventions for COPD. Only four trials reported clinical differences between the intervention and control groups, and these differences were in a range of outcomes, including physical function, QoL, physical activity, and dypsnea19,21,25,31. This apparent lack of impact may be from the small size of the studies, with 8 of the 13 trials reporting a sample size of fewer than 100 participants13,14,15,17,18,23,25,31. In addition, the extent to which the measures used in these studies were sensitive to change is unclear.
Hanlon et al. conducted a metareview of telehealth trials across multiple health conditions, including COPD, diabetes, cancer, and heart failure32. Their findings suggest that the evidence base is more developed in diabetes and heart failure and more intensive and multifaceted interventions associated with greater improvements in asthma, diabetes, and heart failure. Building on published reviews focused on COPD, our findings also report on QoL, self-efficacy, fatigue, anxiety, and depression, as well as exacerbations, physical function, and physical activity. In addition, we provide an in-depth description of the interventions within the included trials.
The results from our pooled data meta-analysis do not identify a statistically significant effect on measures of physical function or QoL. Previous meta-analyses have identified no differences in physical function (using the 6-minute walk test)10, dyspnea10, and average days of hospitalization9, but have noted that the intervention arm was favored for physical activity10 and a lower risk of hospital admissions9.
Looking beyond the effectiveness of the intervention for clinical outcomes, it is possible that there are efficiency and organizational benefits of digital and telehealth care compared with more traditional models of care. None of the studies included in this review reported service outcomes.
The trial interventions identified in our review focused on varying components of COPD self-management, including monitoring symptoms, encouraging lifestyle changes (such as increases in physical activity or exercise), and hosting educational material concerning COPD. Some of the trials explored ease of use, feasibility, and accessibility of the technologies. Aligning with this heterogeneity is the variety of outcome measures used to assess the effectiveness of the intervention. This review highlights the number of outcome measures used and variation in which the tool was used for data collection between studies.
Our findings and the challenges encountered in synthesizing the evidence from these trials highlight the importance of developing a minimum and standardized set of clinically important core-outcome measures to allow comparison of trials involving people with COPD. This would be in line with minimum reporting guidelines for other areas of clinical speciality, including rheumatology33. In practice, the use of mobile device applications to support self-management may have some negative effects. For example, a patient might be falsely reassured if they feel their data were being monitored by a healthcare professional. On the other hand, the data can supplement routine care with information about variation in symptoms and clinical markers of the condition. From a policy perspective, the economic cost of telehealth for chronic disease is high (£92,000/QALY), which restricts its implementation in the majority of healthcare settings34.
In conclusion, this systematic review demonstrates that there are a number of trials being conducted in this area of COPD. However, there is insufficient evidence to date to suggest that mobile device applications are effective for the self-management of COPD over usual care. This may in part be due to a limited ability for data to be pooled, owing to marked variation in methodology and reporting of outcome measures. Future efforts to standardize the outcomes used in this area of research are encouraged to increase the comparability of future trials.
Methods
Registration
The review was registered on the International Prospective Register of Systematic Reviews (PROSPERO reference number: CRD42019124232).
Eligibility criteria
Randomized controlled trials of adults with a clinical diagnosis of COPD were included where the intervention group received a mobile device application to support their COPD self-management. A mobile device application was defined as a contained program that served a specific function relating to COPD and personal health on a portable, electronic device (including smartphones and tablet computers). This definition is in line with previous systematic reviews on the topic11,12. For the purpose of inclusion, self-management was defined as patient management of their personal symptoms and medication regimes related to the condition, as well as coping with the emotional and lifestyle impacts of the condition35,36. Studies were eligible where the comparator group received usual care only. Outcomes included but were not restricted to exacerbations, QoL, physical function, physical activity, and dyspnea.
Information sources and search
Medline, EMBASE, Cochrane Library, CINAHL, and the Science Citation Index were searched from inception to 12th April 2019 following the methods recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines37. Full search strategies are included in Supplementary Methods. The search algorithm focused on keywords relating to ‘COPD’, ‘mobile phone application’, and ‘self-management’ and included interventions with or without healthcare professional input.
Study selection
The resulting citations were imported into the web-based Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia). Screening of titles and abstracts was completed by two authors independently (G.S. and M.W.). In the event of disagreement, two further reviewers (L.A. and A.F.) decided their eligibility. Subsequently, full-text screening was conducted by two authors independently (G.S. and M.W.). Any disagreements were resolved following discussion with the other reviewers (L.A. and A.F.). The reference lists of the included trials were also screened to identify any additional potentially eligible trials.
Data collection process
Extraction forms were used to capture the following data: lead author, year, country, trial setting, sample size, age, sex, lung function, primary and secondary outcomes, duration of intervention and study, as well as the main findings. Data extraction was completed independently by two authors (G.S. and M.W.), and any disagreements were resolved through discussion. When data were not directly identifiable within text or tables, authors were contacted or Microsoft Paint (Microsoft, Washington, USA) was used to extract values from graphs. The graphical summaries were captured by screenshot and copy-pasted into the software. No correction for rotation was required. Horizontal lines were inserted across from the center of the datapoints of interest to the point of intersection on the y-axis. The y-axis was segmented into smaller increments, marked by adding small lines to the axis, until a value could be extracted to 1 decimal place. The values were extracted from the original y-axis scale, meaning the x and y positions were not translated. Two authors (G.S. and M.W.) independently looked at the graphs to identify the value of interest. In the event any disagreements were identified, G.S. and M.W. reassessed the graphs and agreed on a value.
We subsequently replicated the data extraction using web plot digitizer software (Automeris version 3.9, https://automeris.io/WebPlotDigitizer/). The graphical summaries were captured by screenshot and saved as a PNG file before being uploaded to the web-based plot digitizer software. No correction for rotation was required. Once uploaded, two anchoring points were assigned to each axis: the highest and lowest value on the y-axis and baseline and follow-up for the x-axis. Values reflecting these anchoring points were declared. The datapoints were selected using the center of each point to 14 decimal places, and the acquired data were recorded in the form of coordinates that aligned with the scales in the original graphs.
Risk of bias assessment
The included trials were assessed for potential bias at study level using the Cochrane risk of bias tool38. Two authors (G.S. and M.W.) independently completed the assessment of bias, and any disagreements were resolved through discussion with the other reviewers (L.A. and A.F.).
Synthesis of data
The results were converted to mean (standard deviation) when possible; otherwise data were reported as median (lower to upper quartile). A pragmatic decision was made to include outcome measures reported by four or more trials in the main table and those reported less frequently in the text. Where the duration of intervention period and study duration differed, data were extracted for the end of the observation period. Outcomes were grouped together where different measures were used, for example, where different scales for QoL measurement were used. The total scores from the QoL measurement tools were extracted when these were reported; otherwise individual component scores were extracted. Similarly, exacerbations that were treated in the community were grouped, to include self-reported exacerbations (where a participant may have initiated a rescue pack), alongside exacerbations that were managed by primary care teams. Measures of physical activity were included in the summary table if these were objectively measured; self-report of physical activity was not included.
Synthesis of results
Meta-analysis was carried out using Review Manager (Review Manager [RevMan] version 5.3, Cochrane Collaboration, Copenhagen, Denmark). A difference-in-difference random effect analysis was used to help control for differences between trials, and to limit the impact of heterogeneity. Trials were weighted by sample size, and 95% confidence intervals were reported around point estimates. Measures were selected for inclusion if they were reported by at least three trials to align with the recent Cochrane review12. For continuous data with consistent units of measurements (such as the 6-minute walk performance in meters), the mean difference in change between baseline and follow-up measurements was calculated. In instances where continuous data were inconsistent between trials (i.e., multiple questionnaires with varying scales used to measure QoL), the standardized mean differences between timepoints were calculated. Back-translation of the standardized mean difference for each scale was conducted to the original scale, to present a mean difference for each QoL instrument to give information of the clinical significance of this difference. Where change in standard deviation was not reported by individual trials, the standard deviation for changes from baseline was imputed by calculating a correlation coefficient from trials reporting a change in standard deviation. If the data were not reported, authors were contacted to access this information. The I2 statistic was used to estimate heterogeneity. Cochrane recommendations for interpreting the I2 statistic are as follows: 30–60% may represent moderate heterogeneity, 50–90% may represent substantial heterogeneity, and 75–100% may represent considerable heterogeneity39. No funnel plot was produced as it is not recommended for meta-analyses with fewer than 10 trials40.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All data generated or analyzed during this study are included in this published article and Supplementary Material files.
Code availability
No custom code or mathematical algorithm were used.
References
World Health Organisation. The Global Burden of Disease. (2008) https://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf?ua=1.
Mathers, C. D. & Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 3, e442 (2006).
Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. 2018 report (2018).
McLean, S. et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci. Rep. 6, 1–10 (2016).
Foundation, B. L. Estimating the Economic Burden Of Respiratory Illness in the UK blf Estimating the Economic Burden of Respiratory Illness in the UK, British Lung Foundation (2014).
Ståhl, E. et al. Health-related quality of life is related to COPD disease severity. Health Qual. Life Outcomes 3, 56 (2005).
Bourbeau, J. et al. Reduction of hospital utilization in patients with chronic obstructive pulmonary disease: a disease-specific self-management intervention. Arch. Intern. Med. 163, 585–591 (2003).
Williams, V., Price, J., Hardinge, M., Tarassenko, L. & Farmer, A. Using a mobile health application to support self-management in COPD: a qualitative study. Br. J. Gen. Pract. 64, e392–e400 (2014).
Yang, F., Wang, Y., Yang, C., Hu, H. & Xiong, Z. Mobile health applications in self-management of patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis of their efficacy. BMC Pulm. Med. 18, 147 (2018).
Lundell, S., Holmner, Å., Rehn, B., Nyberg, A. & Wadell, K. Telehealthcare in COPD: a systematic review and meta-analysis on physical outcomes and dyspnea. Respir. Med. 109, 11–26 (2015).
Alwashmi, M. et al. The effect of smartphone interventions on patients with chronic obstructive pulmonary disease exacerbations: a systematic review and meta-analysis. JMIR mHealth uHealth 4, e105 (2016).
McCabe, C., McCann, M. & Brady, A. M. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 5, CD011425 (2017).
MG Halpin, D. et al. A randomised controlled trial of the effect of automated interactive calling combined with a health risk forecast on frequency and severity of exacerbations of COPD assessed clinically and using EXACT PRO. Prim. Care Respir. J. 20, 324–331 (2011).
Chau, J. P.-C. et al. A feasibility study to investigate the acceptability and potential effectiveness of a telecare service for older people with chronic obstructive pulmonary disease. Int. J. Med. Inform. 81, 674–682 (2012).
Nguyen, H. Q. et al. Internet-based dyspnea self-management support for patients with chronic obstructive pulmonary disease. J. Pain Symptom Manag. 46, 43–55 (2013).
Pinnock, H. et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: researcher blind, multicentre, randomised controlled trial. BMJ 347, f6070 (2013).
Tabak, M., Vollenbroek-Hutten, M. M., van der Valk, P. D., van der Palen, J. & Hermens, H. J. A telerehabilitation intervention for patients with chronic obstructive pulmonary disease: a randomized controlled pilot trial. Clin. Rehabil. 28, 582–591 (2014).
Tabak, M., Brusse-Keizer, M., van der Valk, P., Hermens, H. & Vollenbroek-Hutten, M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int. J. Chron. Obstruct. Pulmon. Dis. 9, 935–944 (2014).
van der Weegen, S. et al. It’s life! Mobile and web-based monitoring and feedback tool embedded in primary care increases physical activity: a cluster randomized controlled trial. J. Med. Internet Res. 17, e184 (2015).
Vorrink, S. N. W., Kort, H. S. M., Troosters, T., Zanen, P. & Lammers, J.-W. J. Efficacy of an mHealth intervention to stimulate physical activity in COPD patients after pulmonary rehabilitation. Eur. Respir. J. 48, 1019–1029 (2016).
Demeyer, H. et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax https://doi.org/10.1136/thoraxjnl-2016-209026 (2015).
Farmer, A. et al. Self-management support using a digital health system compared with usual care for chronic obstructive pulmonary disease: randomized controlled trial. J. Med. Internet Res. 19, e144 (2017).
Orme, M. W. et al. Findings of the chronic obstructive pulmonary disease-sitting and exacerbations trial (COPD-SEAT) in reducing sedentary time using wearable and mobile technologies with educational support: randomized controlled feasibility trial. JMIR mHealth uHealth 6, e84 (2018).
Wang, T. & Yang, Q. Effect study on improving self-management of patients with chronic obstructive pulmonary disease based on APP. Chin. Nurs. Res. 32, 3121–3124 (2018).
Liu, W.-T. et al. Efficacy of a cell phone-based exercise programme for COPD. Eur. Respir. J. 32, 651–659 (2008).
Seemungal, T. A. R. et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 157, 1418–1422 (1998).
Soler-Cataluña, J. J. et al. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 60, 925–931 (2005).
Miłkowska-Dymanowska, J., Białas, A. J., Obrębski, W., Górski, P. & Piotrowski, W. J. A pilot study of daily telemonitoring to predict acute exacerbation in chronic obstructive pulmonary disease. Int. J. Med. Inform. 116, 46–51 (2018).
Shah, S. A. et al. Personalized alerts for patients with COPD using pulse oximetry and symptom scores. In 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 3164–3167 (IEEE, 2014).
Fernandez-Granero, M. A., Sanchez-Morillo, D. & Leon-Jimenez, A. An artificial intelligence approach to early predict symptom-based exacerbations of COPD. Biotechnol. Biotechnol. Equip. 32, 778–784 (2018).
Zhang, Q. et al. Disease knowledge level is a noteworthy risk factor of anxiety and depression in patients with chronic obstructive pulmonary disease: a cross-sectional study. BMC Pulm. Med. 14, 92 (2014).
Hanlon, P. et al. Telehealth interventions to support self-management of long-term conditions: a systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J. Med. Internet Res. 19, e172 (2017).
Tugwell, P. et al. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials 8, 38 (2007).
Henderson, C. et al. Cost effectiveness of telehealth for patients with long term conditions (Whole Systems Demonstrator telehealth questionnaire study): nested economic evaluation in a pragmatic, cluster randomised controlled trial. BMJ 22, 346 (2013).
Barlow, J., Wright, C., Sheasby, J., Turner, A. & Hainsworth, J. Self-management approaches for people with chronic conditions: a review. Patient Educ. Couns. 48, 177–187 (2002).
Glasgow, R. E., Davis, C. L., Funnell, M. M. & Beck, A. Implementing practical interventions to support chronic illness self-management. Jt. Comm. J. Qual. Saf. 29, 563–574 (2003).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G., PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6, e1000097 (2009).
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J. & Welch, V. A. (eds) Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane. (2019). Available from www.training.cochrane.org/handbook.
Cochrane Collaboration. 9.5.2 Identifying and Measuring Heterogeneity. https://handbook-5-1.cochrane.org/chapter_9/9_5_2_identifying_and_measuring_heterogeneity.htm. Accessed 30 Oct 2019.
Sterne, J. A. C. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002 (2011).
Acknowledgements
The authors would like to thank Ms. Ran Xu for her support in translating one of the papers. A.J.F. is a NIHR Senior Investigator. This project is supported by the NIHR Oxford Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
G.S. made substantial contributions to the conception of the work, acquisition, analysis, and interpretation of data for the work, drafted the work, approved the final version to be published, and agrees to be accountable for all aspects of the work. M.E.W. made substantial contributions to the acquisition, analysis, and interpretation of data for the work, as well as revised the work critically for important intellectual content, approved the final version to be published, and agrees to be accountable for all aspects of the work. L.C.A. made substantial contributions to the acquisition, analysis, and interpretation of data for the work, as well as revised the work critically for important intellectual content, approved the final version to be published, and agrees to be accountable for all aspects of the work. A.J.F. made substantial contributions to the conception of the work and interpretation of data, revised the work critically for important intellectual content, approved the final version to be published, and agrees to be accountable for all aspects of the work. N.R. made substantial contributions to the acquisition of data for the work, as well as revised the work critically for important intellectual content, approved the final version to be published, and agrees to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaw, G., Whelan, M.E., Armitage, L.C. et al. Are COPD self-management mobile applications effective? A systematic review and meta-analysis. npj Prim. Care Respir. Med. 30, 11 (2020). https://doi.org/10.1038/s41533-020-0167-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41533-020-0167-1
This article is cited by
-
Feasibility of a wearable self-management application for patients with COPD at home: a pilot study
BMC Medical Informatics and Decision Making (2024)
-
An umbrella review of effectiveness and efficacy trials for app-based health interventions
npj Digital Medicine (2023)
-
Mobile health applications for self-management in chronic lung disease: a systematic review
Network Modeling Analysis in Health Informatics and Bioinformatics (2023)
-
Participatory methods in a digital setting: experiences from the co-creation of an eHealth tool for people with chronic obstructive pulmonary disease
BMC Medical Informatics and Decision Making (2022)
-
Randomized feasibility trial of the Scleroderma Patient-centered Intervention Network Self-Management (SPIN-SELF) Program
Pilot and Feasibility Studies (2022)