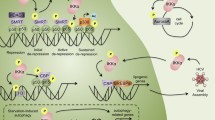
Abstract
Disruption of tissue function activates cellular stress which triggers a number of mechanisms that protect the tissue from further damage. These mechanisms involve a number of homeostatic modules, which are regulated at the level of gene expression by the transactivator NF-κB. This transcription factor shifts between activation and repression of discrete, cell-dependent gene expression clusters. Some of its target genes provide feedback to NF-κB itself, thereby strengthening the inflammatory response of the tissue and later terminating inflammation to facilitate restoration of tissue homeostasis. Disruption of key feedback modules for NF-κB in certain cell types facilitates the survival of clones with genomic aberrations, and protects them from being recognized and eliminated by the immune system, to enable thereby carcinogenesis.


Similar content being viewed by others
References
Singh H, Sen R, Baltimore D, Sharp PA (1986) A nuclear factor that binds to a conserved sequence motif in transcriptional control elements of immunoglobulin genes. Nature 319:154–158. https://doi.org/10.1038/319154a0
Sen R, Baltimore D (1986) Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46:705–716
Sha WC, Liou HC, Tuomanen EI, Baltimore D (1995) Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 80:321–330
Vlahopoulos SA, Cen O, Hengen N et al (2015) Dynamic aberrant NF-κB spurs tumorigenesis: a new model encompassing the microenvironment. Cytokine Growth Factor Rev 26:389–403. https://doi.org/10.1016/j.cytogfr.2015.06.001
Park MH, Hong JT (2016) Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. https://doi.org/10.3390/cells5020015
Luo K (2017) Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a022137
Pan D, Lin X (2013) Epithelial growth factor receptor-activated nuclear factor κB signaling and its role in epithelial growth factor receptor-associated tumors. Cancer J Sudbury Mass 19:461–467. https://doi.org/10.1097/PPO.0000000000000001
Vanden Berghe W, Dijsselbloem N, Vermeulen L et al (2006) Attenuation of mitogen- and stress-activated protein kinase-1-driven nuclear factor-kappaB gene expression by soy isoflavones does not require estrogenic activity. Cancer Res 66:4852–4862. https://doi.org/10.1158/0008-5472.CAN-05-2957
Kourdis PD, Palasantza AG, Goussis DA (2013) Algorithmic asymptotic analysis of the NF-κB signaling system. Comput Math Appl 65:1516–1534. https://doi.org/10.1016/j.camwa.2012.11.004
Chishti AA, Baumstark-Khan C, Koch K et al (2018) Linear energy transfer modulates radiation-induced NF-kappa B activation and expression of its downstream target genes. Radiat Res 189:354–370. https://doi.org/10.1667/RR14905.1
Li D, Sun J, Liu W et al (2016) Rig-G is a growth inhibitory factor of lung cancer cells that suppresses STAT3 and NF-κB. Oncotarget 7:66032–66050. https://doi.org/10.18632/oncotarget.11797
Vlahopoulos SA (2017) Aberrant control of NF-κB in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: molecular mode. Cancer Biol Med 14:254–270. https://doi.org/10.20892/j.issn.2095-3941.2017.0029
Saccani S, Pantano S, Natoli G (2003) Modulation of NF-kappaB activity by exchange of dimers. Mol Cell 11:1563–1574
Christian F, Smith EL, Carmody RJ (2016) The regulation of NF-κB subunits by phosphorylation. Cells. https://doi.org/10.3390/cells5010012
Brignall R, Moody AT, Mathew S, Gaudet S (2019) Considering abundance, affinity, and binding site availability in the NF-κB target selection puzzle. Front Immunol 10:609. https://doi.org/10.3389/fimmu.2019.00609
Li X, Luo R, Chen R et al (2014) Cleavage of IκBα by calpain induces myocardial NF-κB activation, TNF-α expression, and cardiac dysfunction in septic mice. Am J Physiol Heart Circ Physiol 306:H833–843. https://doi.org/10.1152/ajpheart.00893.2012
Varisli L, Cen O, Vlahopoulos S (2019) Dissecting pharmacological effects of Chloroquine in cancer treatment: interference with inflammatory signaling pathways. Immunology. https://doi.org/10.1111/imm.13160
Grinberg-Bleyer Y, Caron R, Seeley JJ et al (1950) (2018) The alternative NF-κB pathway in regulatory T cell homeostasis and suppressive function. J Immunol Baltim MD 200:2362–2371. https://doi.org/10.4049/jimmunol.1800042
Thu YM, Richmond A (2010) NF-κB inducing kinase: a key regulator in the immune system and in cancer. Cytokine Growth Factor Rev 21:213–226. https://doi.org/10.1016/j.cytogfr.2010.06.002
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 27:693–733. https://doi.org/10.1146/annurev.immunol.021908.132641
Chatterjee B, Roy P, Sarkar UA et al (2019) Immune differentiation regulator p100 tunes NF-κB responses to TNF. Front Immunol 10:997. https://doi.org/10.3389/fimmu.2019.00997
Jamaluddin M, Wang S, Boldogh I et al (2007) TNF-alpha-induced NF-kappaB/RelA Ser(276) phosphorylation and enhanceosome formation is mediated by an ROS-dependent PKAc pathway. Cell Signal 19:1419–1433. https://doi.org/10.1016/j.cellsig.2007.01.020
Rokavec M, Öner MG, Hermeking H (2016) lnflammation-induced epigenetic switches in cancer. Cell Mol Life Sci CMLS 73:23–39. https://doi.org/10.1007/s00018-015-2045-5
Zhong Z, Umemura A, Sanchez-Lopez E et al (2016) NF-κB restricts inflammasome activation via elimination of damaged mitochondria. Cell 164:896–910. https://doi.org/10.1016/j.cell.2015.12.057
Su S, Chen J, Yao H et al (2018) CD10+GPR77+ cancer-associated fibroblasts promote cancer formation and chemoresistance by sustaining cancer stemness. Cell 172:841–856.e16. https://doi.org/10.1016/j.cell.2018.01.009
Yang D, Kim J (2019) Mitochondrial retrograde signalling and metabolic alterations in the tumour microenvironment. Cells. https://doi.org/10.3390/cells8030275
Bansal A, Henao-Mejia J, Simmons RA (2018) Immune system: an emerging player in mediating effects of endocrine disruptors on metabolic health. Endocrinology 159:32–45. https://doi.org/10.1210/en.2017-00882
Fischer C, Mamillapalli R, Goetz LG et al (2016) Bisphenol A (BPA) exposure in utero leads to immunoregulatory cytokine dysregulation in the mouse mammary gland: a potential mechanism programming breast cancer risk. Horm Cancer 7:241–251. https://doi.org/10.1007/s12672-016-0254-5
Huang F-M, Chang Y-C, Lee S-S et al (2019) Expression of pro-inflammatory cytokines and mediators induced by Bisphenol A via ERK-NFκB and JAK1/2-STAT3 pathways in macrophages. Environ Toxicol 34:486–494. https://doi.org/10.1002/tox.22702
Spiropoulos A, Goussetis E, Margeli A et al (2010) Effect of inflammation induced by prolonged exercise on circulating erythroid progenitors and markers of erythropoiesis. Clin Chem Lab Med 48:199–203. https://doi.org/10.1515/CCLM.2010.034
Willenberg HS, Stratakis CA, Marx C et al (1998) Aberrant interleukin-1 receptors in a cortisol-secreting adrenal adenoma causing Cushing’s syndrome. N Engl J Med 339:27–31. https://doi.org/10.1056/NEJM199807023390105
Vlahopoulos S, Boldogh I, Casola A, Brasier AR (1999) Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 94:1878–1889
Wajant H, Pfizenmaier K, Scheurich P (2003) Tumor necrosis factor signaling. Cell Death Differ 10:45–65. https://doi.org/10.1038/sj.cdd.4401189
Doi TS, Marino MW, Takahashi T et al (1999) Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc Natl Acad Sci USA 96:2994–2999. https://doi.org/10.1073/pnas.96.6.2994
Xu C, Wu X, Zhang X et al (1950) (2018) Embryonic lethality and host immunity of RelA-deficient mice are mediated by both apoptosis and necroptosis. J Immunol Baltim MD 200:271–285. https://doi.org/10.4049/jimmunol.1700859
Murugesan M, Premkumar K (2018) Hypoxia stimulates microenvironment in human embryonic stem cell through inflammatory signalling: an integrative analysis. Biochem Biophys Res Commun 498:437–444. https://doi.org/10.1016/j.bbrc.2018.02.194
Sionov RV, Vlahopoulos SA, Granot Z (2015) Regulation of bim in health and disease. Oncotarget 6:23058–23134. https://doi.org/10.18632/oncotarget.5492
Shen H, Wu N, Nanayakkara G et al (2019) Co-signaling receptors regulate T-cell plasticity and immune tolerance. Front Biosci Landmark Ed 24:96–132
Sionov RV, Fridlender ZG, Granot Z (2015) The multifaceted roles neutrophils play in the tumor microenvironment. Cancer Microenviron 8:125–158. https://doi.org/10.1007/s12307-014-0147-5
Spath P, Tisato V, Gianesini S et al (2017) The calendar of cytokines: Seasonal variation of circulating cytokines in chronic venous insufficiency. JRSM Cardiovasc Dis 6:2048004017729279. https://doi.org/10.1177/2048004017729279
Jezierska A, Kolosova IA, Verin AD (2011) Toll like receptors signaling pathways as a target for therapeutic interventions. Curr Signal Transduct Ther 6:428–440. https://doi.org/10.2174/157436211797483930
Adamczak DM (2017) The role of toll-like receptors and vitamin D in cardiovascular diseases: a review. Int J Mol Sci. https://doi.org/10.3390/ijms18112252
Pan L, Zhu B, Hao W et al (2016) Oxidized guanine base lesions function in 8-oxoguanine DNA glycosylase-1-mediated epigenetic regulation of nuclear factor κB-driven gene expression. J Biol Chem 291:25553–25566. https://doi.org/10.1074/jbc.M116.751453
Hao W, Qi T, Pan L et al (2018) Effects of the stimuli-dependent enrichment of 8-oxoguanine DNA glycosylase1 on chromatinized DNA. Redox Biol 18:43–53. https://doi.org/10.1016/j.redox.2018.06.002
Hellweg CE, Spitta LF, Koch K et al (2018) The role of the nuclear factor κB pathway in the cellular response to low and high linear energy transfer radiation. Int J Mol Sci. https://doi.org/10.3390/ijms19082220
Zhang Y, Huang W (2018) Transforming growth factor β1 (TGF-β1)-stimulated integrin-linked kinase (ILK) regulates migration and epithelial-mesenchymal transition (EMT) of human lens epithelial cells via nuclear factor κB (NF-κB). Med Sci Monit 24:7424–7430. https://doi.org/10.12659/MSM.910601
Bekeredjian-Ding I, Jego G (2009) Toll-like receptors–sentries in the B-cell response. Immunology 128:311–323. https://doi.org/10.1111/j.1365-2567.2009.03173.x
Bekeredjian-Ding I, Doster A, Schiller M et al (1950) (2008) TLR9-activating DNA up-regulates ZAP70 via sustained PKB induction in IgM+ B cells. J Immunol Baltim MD 181:8267–8277. https://doi.org/10.4049/jimmunol.181.12.8267
Gerondakis S, Grumont RJ, Banerjee A (2007) Regulating B-cell activation and survival in response to TLR signals. Immunol Cell Biol 85:471–475. https://doi.org/10.1038/sj.icb.7100097
Marshall-Clarke S, Downes JE, Haga IR et al (2007) Polyinosinic acid is a ligand for toll-like receptor 3. J Biol Chem 282:24759–24766. https://doi.org/10.1074/jbc.M700188200
Richards S, Watanabe C, Santos L et al (2008) Regulation of B-cell entry into the cell cycle. Immunol Rev 224:183–200. https://doi.org/10.1111/j.1600-065X.2008.00652.x
Takahashi K, Kawai T, Kumar H et al (1950) (2006) Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J Immunol Baltim MD 176:4520–4524. https://doi.org/10.4049/jimmunol.176.8.4520
Sedger LM, McDermott MF (2014) TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev 25:453–472. https://doi.org/10.1016/j.cytogfr.2014.07.016
Szebeni GJ, Nagy LI, Berkó A et al (2019) The anti-inflammatory role of Mannich curcuminoids; special focus on colitis. Mol Basel Switz. https://doi.org/10.3390/molecules24081546
Xu W, Santini PA, Matthews AJ et al (1950) (2008) Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J Immunol Baltim MD 181:276–287. https://doi.org/10.4049/jimmunol.181.1.276
Puimège L, Libert C, Van Hauwermeiren F (2014) Regulation and dysregulation of tumor necrosis factor receptor-1. Cytokine Growth Factor Rev 25:285–300. https://doi.org/10.1016/j.cytogfr.2014.03.004
Arjuman A, Chandra NC (2015) Differential pro-inflammatory responses of TNF-α receptors (TNFR1 and TNFR2) on LOX-1 signalling. Mol Biol Rep 42:1039–1047. https://doi.org/10.1007/s11033-014-3841-y
Gane JM, Stockley RA, Sapey E (2016) TNF-α autocrine feedback loops in human monocytes: the pro- and anti-inflammatory roles of the TNF-α receptors support the concept of selective TNFR1 blockade in vivo. J Immunol Res 2016:1079851. https://doi.org/10.1155/2016/1079851
Nowak DE, Tian B, Jamaluddin M et al (2008) RelA Ser276 phosphorylation is required for activation of a subset of NF-kappaB-dependent genes by recruiting cyclin-dependent kinase 9/cyclin T1 complexes. Mol Cell Biol 28:3623–3638. https://doi.org/10.1128/MCB.01152-07
Ijaz T, Wakamiya M, Sun H et al (2016) Generation and characterization of a novel transgenic mouse harboring conditional nuclear factor-kappa B/RelA knockout alleles. BMC Dev Biol 16:32. https://doi.org/10.1186/s12861-016-0135-8
Wang CY, Mayo MW, Korneluk RG et al (1998) NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680–1683. https://doi.org/10.1126/science.281.5383.1680
Sung N-Y, Yang M-S, Song D-S et al (2013) The procyanidin trimer C1 induces macrophage activation via NF-κB and MAPK pathways, leading to Th1 polarization in murine splenocytes. Eur J Pharmacol 714:218–228. https://doi.org/10.1016/j.ejphar.2013.02.059
Rius J, Guma M, Schachtrup C et al (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453:807–811. https://doi.org/10.1038/nature06905
Chal J, Pourquié O (2017) Making muscle: skeletal myogenesis in vivo and in vitro. Dev Camb Engl 144:2104–2122. https://doi.org/10.1242/dev.151035
Gabut M, Bourdelais F, Durand S (2020) Ribosome and translational control in stem cells. Cells. https://doi.org/10.3390/cells9020497
Tosenberger A, Gonze D, Bessonnard S et al (2017) A multiscale model of early cell lineage specification including cell division. NPJ Syst Biol Appl 3:16. https://doi.org/10.1038/s41540-017-0017-0
O’Connor KC (2019) Molecular profiles of cell-to-cell variation in the regenerative potential of mesenchymal stromal cells. Stem Cells Int 2019:5924878. https://doi.org/10.1155/2019/5924878
Lenardo MJ, Kuang A, Gifford A, Baltimore D (1989) Purified bovine NF-kappa B recognizes regulatory sequences in multiple genes expressed during activation of T- and B-lymphocytes. Haematol Blood Transfus 32:411–415
Liou HC, Baltimore D (1993) Regulation of the NF-kappa B/rel transcription factor and I kappa B inhibitor system. Curr Opin Cell Biol 5:477–487
Copland JA, Sheffield-Moore M, Koldzic-Zivanovic N et al (2009) Sex steroid receptors in skeletal differentiation and epithelial neoplasia: is tissue-specific intervention possible? BioEssays News Rev Mol Cell Dev Biol 31:629–641. https://doi.org/10.1002/bies.200800138
Mittal A, Papa S, Franzoso G (1950) Sen R (2006) NF-kappaB-dependent regulation of the timing of activation-induced cell death of T lymphocytes. J Immunol Baltim MD 176:2183–2189
Moschovi M, Critselis E, Cen O et al (2015) Drugs acting on homeostasis: challenging cancer cell adaptation. Expert Rev Anticancer Ther 15:1405–1417. https://doi.org/10.1586/14737140.2015.1095095
Oliveira-Marques V, Marinho HS, Cyrne L, Antunes F (2009) Role of hydrogen peroxide in NF-kappaB activation: from inducer to modulator. Antioxid Redox Signal 11:2223–2243. https://doi.org/10.1089/ars.2009.2601
Yazdani S, Bansal R, Prakash J (2017) Drug targeting to myofibroblasts: implications for fibrosis and cancer. Adv Drug Deliv Rev 121:101–116. https://doi.org/10.1016/j.addr.2017.07.010
Collignon E, Canale A, Al Wardi C et al (2018) Immunity drives TET1 regulation in cancer through NF-κB. Sci Adv 4:eaap7309. https://doi.org/10.1126/sciadv.aap7309
Fischer JC, Otten V, Kober M et al (1950) (2017) A20 restrains thymic regulatory T cell development. J Immunol Baltim MD 199:2356–2365. https://doi.org/10.4049/jimmunol.1602102
Brantley DM, Chen CL, Muraoka RS et al (2001) Nuclear factor-kappaB (NF-kappaB) regulates proliferation and branching in mouse mammary epithelium. Mol Biol Cell 12:1445–1455. https://doi.org/10.1091/mbc.12.5.1445
Romagnoli M, Belguise K, Yu Z et al (2012) Epithelial-to-mesenchymal transition induced by TGF-β1 is mediated by Blimp-1-dependent repression of BMP-5. Cancer Res 72:6268–6278. https://doi.org/10.1158/0008-5472.CAN-12-2270
Lindfors PH, Voutilainen M, Mikkola ML (2013) Ectodysplasin/NF-κB signaling in embryonic mammary gland development. J Mammary Gland Biol Neoplasia 18:165–169. https://doi.org/10.1007/s10911-013-9277-5
Segev DL, Hoshiya Y, Hoshiya M et al (2002) Mullerian-inhibiting substance regulates NF-kappa B signaling in the prostate in vitro and in vivo. Proc Natl Acad Sci USA 99:239–244. https://doi.org/10.1073/pnas.221599298
Liotti F, Collina F, Pone E et al (2017) Interleukin-8, but not the related chemokine CXCL1, sustains an autocrine circuit necessary for the properties and functions of thyroid cancer stem cells. Stem Cells Dayt Ohio 35:135–146. https://doi.org/10.1002/stem.2492
Galdiero MR, Varricchi G, Loffredo S et al (2018) Potential involvement of neutrophils in human thyroid cancer. PLoS ONE 13:e0199740. https://doi.org/10.1371/journal.pone.0199740
Zou Z-W, Liu T, Li Y et al (2018) Melatonin suppresses thyroid cancer growth and overcomes radioresistance via inhibition of p65 phosphorylation and induction of ROS. Redox Biol 16:226–236. https://doi.org/10.1016/j.redox.2018.02.025
Sionov RV, Assi S, Gershkovitz M et al (2015) Isolation and characterization of neutrophils with anti-tumor properties. J Vis Exp JoVE. https://doi.org/10.3791/52933
Chung HY, Kim DH, Lee EK et al (2019) Redefining chronic inflammation in aging and age-related diseases: proposal of the senoinflammation concept. Aging Dis 10:367–382. https://doi.org/10.14336/AD.2018.0324
Zhang G, Li J, Purkayastha S et al (2013) Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497:211–216. https://doi.org/10.1038/nature12143
Listwak SJ, Rathore P, Herkenham M (2013) Minimal NF-κB activity in neurons. Neuroscience 250:282–299. https://doi.org/10.1016/j.neuroscience.2013.07.013
Liu C, Zhang F, Liu H, Wei F (2018) NF-kB mediated CX3CL1 activation in the dorsal root ganglion contributes to the maintenance of neuropathic pain induced in adult male Sprague Dawley rats1. Acta Cir Bras 33:619–628. https://doi.org/10.1590/s0102-865020180070000007
Ferreira ZS, Fernandes PACM, Duma D et al (2005) Corticosterone modulates noradrenaline-induced melatonin synthesis through inhibition of nuclear factor kappa B. J Pineal Res 38:182–188. https://doi.org/10.1111/j.1600-079X.2004.00191.x
Weger M, Diotel N, Dorsemans A-C et al (2017) Stem cells and the circadian clock. Dev Biol 431:111–123. https://doi.org/10.1016/j.ydbio.2017.09.012
Umemura Y, Maki I, Tsuchiya Y et al (2019) Human circadian molecular oscillation development using induced pluripotent stem cells. J Biol Rhythms. https://doi.org/10.1177/0748730419865436
Antonioli M, Rybka J, Carvalho LA (2012) Neuroimmune endocrine effects of antidepressants. Neuropsychiatr Dis Treat 8:65–83. https://doi.org/10.2147/NDT.S16409
Markus RP, Cecon E, Pires-Lapa MA (2013) Immune-pineal axis: nuclear factor κB (NF-kB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int J Mol Sci 14:10979–10997. https://doi.org/10.3390/ijms140610979
Couto-Moraes R, Palermo-Neto J, Markus RP (2009) The immune-pineal axis: stress as a modulator of pineal gland function. Ann N Y Acad Sci 1153:193–202. https://doi.org/10.1111/j.1749-6632.2008.03978.x
Cecon E, Fernandes PA, Pinato L et al (2010) Daily variation of constitutively activated nuclear factor kappa B (NFKB) in rat pineal gland. Chronobiol Int 27:52–67. https://doi.org/10.3109/07420521003661615
Mo M-S, Li H-B, Wang B-Y et al (2013) PI3K/Akt and NF-κB activation following intravitreal administration of 17β-estradiol: neuroprotection of the rat retina from light-induced apoptosis. Neuroscience 228:1–12. https://doi.org/10.1016/j.neuroscience.2012.10.002
Giannoulia-Karantana A, Vlachou A, Polychronopoulou S et al (2006) Melatonin and immunomodulation: connections and potential clinical applications. NeuroImmunoModulation 13:133–144. https://doi.org/10.1159/000097258
Chen X, Zhang J, Chen C (2011) Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults. Neuroscience 178:159–168. https://doi.org/10.1016/j.neuroscience.2011.01.024
Garrido-Gil P, Rodriguez-Pallares J, Dominguez-Meijide A et al (2013) Brain angiotensin regulates iron homeostasis in dopaminergic neurons and microglial cells. Exp Neurol 250:384–396. https://doi.org/10.1016/j.expneurol.2013.10.013
Xu X, Lei Y, Luo J et al (2013) Prevention of β-amyloid induced toxicity in human iPS cell-derived neurons by inhibition of Cyclin-dependent kinases and associated cell cycle events. Stem Cell Res 10:213–227. https://doi.org/10.1016/j.scr.2012.11.005
Chu L-F, Wang W-T, Ghanta VK et al (2008) Ischemic brain cell-derived conditioned medium protects astrocytes against ischemia through GDNF/ERK/NF-kB signaling pathway. Brain Res 1239:24–35. https://doi.org/10.1016/j.brainres.2008.08.087
Huleihel M, Fadlon E, Abuelhija A et al (2013) Glial cell line-derived neurotrophic factor (GDNF) induced migration of spermatogonial cells in vitro via MEK and NF-kB pathways. Differ Res Biol Divers 86:38–47. https://doi.org/10.1016/j.diff.2013.06.005
Tian H, Yao S-T, Yang N-N et al (2017) D4F alleviates macrophage-derived foam cell apoptosis by inhibiting the NF-κB-dependent Fas/FasL pathway. Sci Rep 7:7333. https://doi.org/10.1038/s41598-017-07656-0
Bu Y, Li X, He Y et al (2016) A phosphomimetic mutant of RelA/p65 at Ser536 induces apoptosis and senescence: an implication for tumor-suppressive role of Ser536 phosphorylation. Int J Cancer 138:1186–1198. https://doi.org/10.1002/ijc.29852
Peng Y, Gallagher SF, Landmann R et al (2006) The role of p65 NF-kappaB/RelA in pancreatitis-induced Kupffer cell apoptosis. J Gastrointest Surg 10:837–847. https://doi.org/10.1016/j.gassur.2005.12.013
Xiong J, Wang K, Yuan C et al (2017) Luteolin protects mice from severe acute pancreatitis by exerting HO-1-mediated anti-inflammatory and antioxidant effects. Int J Mol Med 39:113–125. https://doi.org/10.3892/ijmm.2016.2809
Chatterjee B, Banoth B, Mukherjee T et al (2016) Late-phase synthesis of IκBα insulates the TLR4-activated canonical NF-κB pathway from noncanonical NF-κB signaling in macrophages. Sci Signal 9:ra120. https://doi.org/10.1126/scisignal.aaf1129
Castellucci M, Rossato M, Calzetti F et al (2015) IL-10 disrupts the Brd4-docking sites to inhibit LPS-induced CXCL8 and TNF-α expression in monocytes: Implications for chronic obstructive pulmonary disease. J Allergy Clin Immunol 136:781–791.e9. https://doi.org/10.1016/j.jaci.2015.04.023
He X, Zheng Y, Liu S et al (2018) MiR-146a protects small intestine against ischemia/reperfusion injury by down-regulating TLR4/TRAF6/NF-κB pathway. J Cell Physiol 233:2476–2488. https://doi.org/10.1002/jcp.26124
Singh AK, Fechtner S, Chourasia M et al (2019) Critical role of IL-1α in IL-1β-induced inflammatory responses: cooperation with NF-κBp65 in transcriptional regulation. FASEB J 33:2526–2536. https://doi.org/10.1096/fj.201801513R
Ding H-G, Deng Y-Y, Yang R-Q et al (2018) Hypercapnia induces IL-1β overproduction via activation of NLRP3 inflammasome: implication in cognitive impairment in hypoxemic adult rats. J Neuroinflamm 15:4. https://doi.org/10.1186/s12974-017-1051-y
Khan YM, Kirkham P, Barnes PJ, Adcock IM (2014) Brd4 is essential for IL-1β-induced inflammation in human airway epithelial cells. PLoS ONE 9:e95051. https://doi.org/10.1371/journal.pone.0095051
Belkina AC, Nikolajczyk BS (1950) Denis GV (2013) BET protein function is required for inflammation: Brd2 genetic disruption and BET inhibitor JQ1 impair mouse macrophage inflammatory responses. J Immunol Baltim MD 190:3670–3678. https://doi.org/10.4049/jimmunol.1202838
Scott LM, Bryant AH, Rees A et al (2017) Production and regulation of interleukin-1 family cytokines at the materno-fetal interface. Cytokine 99:194–202. https://doi.org/10.1016/j.cyto.2017.07.005
Rizzo FR, Musella A, De Vito F et al (2018) Tumor necrosis factor and Interleukin-1β modulate synaptic plasticity during neuroinflammation. Neural Plast 2018:8430123. https://doi.org/10.1155/2018/8430123
Maurer SV, Williams CL (2017) The cholinergic system modulates memory and hippocampal plasticity via its interactions with non-neuronal cells. Front Immunol 8:1489. https://doi.org/10.3389/fimmu.2017.01489
He Y, Jackman NA, Thorn TL et al (2015) Interleukin-1β protects astrocytes against oxidant-induced injury via an NF-κB-dependent upregulation of glutathione synthesis. Glia 63:1568–1580. https://doi.org/10.1002/glia.22828
Chowdhury T, Allen MF, Thorn TL et al (2018) Interleukin-1β protects neurons against oxidant-induced injury via the promotion of astrocyte glutathione production. Antioxid Basel Switz. https://doi.org/10.3390/antiox7080100
Chaweewannakorn C, Tsuchiya M, Koide M et al (2018) Roles of IL-1α/β in regeneration of cardiotoxin-injured muscle and satellite cell function. Am J Physiol Regul Integr Comp Physiol 315:R90–R103. https://doi.org/10.1152/ajpregu.00310.2017
Zhu J, Huang J, Dai D et al (2019) Recombinant human interleukin-1 receptor antagonist treatment protects rats from myocardial ischemia-reperfusion injury. Biomed Pharmacother Biomed Pharmacother 111:1–5. https://doi.org/10.1016/j.biopha.2018.12.031
Yan C, Gao N, Sun H et al (2016) Targeting imbalance between IL-1β and IL-1 receptor antagonist ameliorates delayed epithelium wound healing in diabetic mouse corneas. Am J Pathol 186:1466–1480. https://doi.org/10.1016/j.ajpath.2016.01.019
Yamanaka K, Nakanishi T, Saito H et al (2014) Persistent release of IL-1s from skin is associated with systemic cardio-vascular disease, emaciation and systemic amyloidosis: the potential of anti-IL-1 therapy for systemic inflammatory diseases. PLoS ONE 9:e104479. https://doi.org/10.1371/journal.pone.0104479
Qu R, Chen X, Wang W et al (2018) Ghrelin protects against osteoarthritis through interplay with Akt and NF-κB signaling pathways. FASEB J 32:1044–1058. https://doi.org/10.1096/fj.201700265R
Liu J, Cao L, Gao X et al (2018) Ghrelin prevents articular cartilage matrix destruction in human chondrocytes. Biomed Pharmacother Biomed Pharmacother 98:651–655. https://doi.org/10.1016/j.biopha.2017.12.050
Bacopoulou F, Athanasopoulos N, Efthymiou V et al (2018) Serum irisin concentrations in lean adolescents with polycystic ovary syndrome. Clin Endocrinol (Oxf) 88:585–591. https://doi.org/10.1111/cen.13555
Mazur-Bialy AI, Pocheć E, Zarawski M (2017) Anti-inflammatory properties of irisin, mediator of physical activity, are connected with TLR4/MyD88 signaling pathway activation. Int J Mol Sci. https://doi.org/10.3390/ijms18040701
Tian B, Widen SG, Yang J et al (2018) The NFκB subunit RELA is a master transcriptional regulator of the committed epithelial-mesenchymal transition in airway epithelial cells. J Biol Chem 293:16528–16545. https://doi.org/10.1074/jbc.RA118.003662
Shen H, Ma J-L, Zhang Y et al (2016) Integrin-linked kinase overexpression promotes epithelial-mesenchymal transition via nuclear factor-κB signaling in colorectal cancer cells. World J Gastroenterol 22:3969–3977. https://doi.org/10.3748/wjg.v22.i15.3969
Park SH, Riley P, Frisch SM (2013) Regulation of anoikis by deleted in breast cancer-1 (DBC1) through NF-κB. Apoptosis Int J Program Cell Death 18:949–962. https://doi.org/10.1007/s10495-013-0847-1
Lin Y, Ukaji T, Koide N, Umezawa K (2018) Inhibition of late and early phases of cancer metastasis by the NF-κB inhibitor DHMEQ derived from microbial bioactive metabolite epoxyquinomicin: a review. Int J Mol Sci. https://doi.org/10.3390/ijms19030729
Deep G, Jain AK, Ramteke A et al (2014) SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin. Mol Cancer 13:37. https://doi.org/10.1186/1476-4598-13-37
Men W, Li W, Zhao J, Li Y (2018) TIFA promotes cell survival and migration in lung adenocarcinoma. Cell Physiol Biochem 47:2097–2108. https://doi.org/10.1159/000491478
Grinberg-Bleyer Y, Oh H, Desrichard A et al (2017) NF-κB c-rel is crucial for the regulatory T cell immune checkpoint in cancer. Cell 170:1096–1108.e13. https://doi.org/10.1016/j.cell.2017.08.004
Vlahopoulos S, Adamaki M, Khoury N et al (2018) Roles of DNA repair enzyme OGG1 in innate immunity and its significance for lung cancer. Pharmacol Ther. https://doi.org/10.1016/j.pharmthera.2018.09.004
Jones VS, Huang R-Y, Chen L-P et al (2016) Cytokines in cancer drug resistance: cues to new therapeutic strategies. Biochim Biophys Acta 1865:255–265. https://doi.org/10.1016/j.bbcan.2016.03.005
Wu T-H, Yeh K-Y, Wang C-H et al (2019) The combination of astragalus membranaceus and Angelica sinensis inhibits lung cancer and Cachexia through its immunomodulatory function. J Oncol 2019:9206951. https://doi.org/10.1155/2019/9206951
Dextraze K, Saha A, Kim D et al (2017) Spatial habitats from multiparametric MR imaging are associated with signaling pathway activities and survival in glioblastoma. Oncotarget 8:112992–113001. https://doi.org/10.18632/oncotarget.22947
Lai X-L, Deng Z-F, Zhu X-G, Chen Z-H (2019) Apc gene suppresses intracranial aneurysm formation and rupture through inhibiting the NF-κB signaling pathway mediated inflammatory response. Biosci Rep. https://doi.org/10.1042/BSR20181909
Liu Q, Shan P, Li H (2019) Gambogic acid prevents angiotensin II-induced abdominal aortic aneurysm through inflammatory and oxidative stress dependent targeting the PI3K/Akt/mTOR and NF-κB signaling pathways. Mol Med Rep 19:1396–1402. https://doi.org/10.3892/mmr.2018.9720
Nalla AK, Gogineni VR, Gupta R et al (2011) Suppression of uPA and uPAR blocks radiation-induced MCP-1 mediated recruitment of endothelial cells in meningioma. Cell Signal 23:1299–1310. https://doi.org/10.1016/j.cellsig.2011.03.011
Ling C, Pouget C, Rech F et al (2016) Endothelial cell hypertrophy and microvascular proliferation in meningiomas are correlated with higher histological grade and shorter progression-free survival. J Neuropathol Exp Neurol 75:1160–1170. https://doi.org/10.1093/jnen/nlw095
Aghajan M, Li N, Karin M (2012) Obesity, autophagy and the pathogenesis of liver and pancreatic cancers. J Gastroenterol Hepatol 27(Suppl 2):10–14. https://doi.org/10.1111/j.1440-1746.2011.07008.x
Daniluk J, Liu Y, Deng D et al (2012) An NF-κB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest 122:1519–1528. https://doi.org/10.1172/JCI59743
Zambirinis CP, Miller G (2017) Cancer manipulation of host physiology: lessons from pancreatic cancer. Trends Mol Med 23:465–481. https://doi.org/10.1016/j.molmed.2017.03.003
Guttridge DC, Albanese C, Reuther JY et al (1999) NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 19:5785–5799. https://doi.org/10.1128/mcb.19.8.5785
Huang Y, Ohtani K, Iwanaga R et al (2001) Direct trans-activation of the human cyclin D2 gene by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 20:1094–1102. https://doi.org/10.1038/sj.onc.1204198
Chopra A, Ferreira-Alves DL, Sirois P, Thirion JP (1992) Cloning of the guinea pig 5-lipoxygenase gene and nucleotide sequence of its promoter. Biochem Biophys Res Commun 185:489–495. https://doi.org/10.1016/0006-291x(92)91651-6
Morri H, Ozaki M, Watanabe Y (1994) 5’-flanking region surrounding a human cytosolic phospholipase A2 gene. Biochem Biophys Res Commun 205:6–11. https://doi.org/10.1006/bbrc.1994.2621
Arkan MC, Hevener AL, Greten FR et al (2005) IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med 11:191–198. https://doi.org/10.1038/nm1185
Revollo JR, Oakley RH, Lu NZ et al (2013) HES1 is a master regulator of glucocorticoid receptor-dependent gene expression. Sci Signal 6:ra103. https://doi.org/10.1126/scisignal.2004389
Hellweg CE (2015) The nuclear factor κB pathway: a link to the immune system in the radiation response. Cancer Lett 368:275–289. https://doi.org/10.1016/j.canlet.2015.02.019
Kan S, Zhang W, Mao J et al (2018) NF-κB activation contributes to parathyroid cell proliferation in chronic kidney disease. J Nephrol 31:941–951. https://doi.org/10.1007/s40620-018-0530-2
Tessier F, Fontaine-Bisson B, Lefebvre J-F et al (2019) Investigating gene-gene and gene-environment interactions in the association between overnutrition and obesity-related phenotypes. Front Genet 10:151. https://doi.org/10.3389/fgene.2019.00151
Takahashi H, Ogata H, Nishigaki R et al (2010) Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell 17:89–97. https://doi.org/10.1016/j.ccr.2009.12.008
Font-Burgada J, Shalapour S, Ramaswamy S et al (2015) Hybrid periportal hepatocytes regenerate the injured liver without giving rise to cancer. Cell 162:766–779. https://doi.org/10.1016/j.cell.2015.07.026
Zhao JL, Baltimore D (2015) Regulation of stress-induced hematopoiesis. Curr Opin Hematol 22:286–292. https://doi.org/10.1097/MOH.0000000000000149
Hou Y, Li F, Karin M, Ostrowski MC (2008) Analysis of the IKKbeta/NF-kappaB signaling pathway during embryonic angiogenesis. Dev Dyn 237:2926–2935. https://doi.org/10.1002/dvdy.21723
Guma M, Stepniak D, Shaked H et al (2011) Constitutive intestinal NF-κB does not trigger destructive inflammation unless accompanied by MAPK activation. J Exp Med 208:1889–1900. https://doi.org/10.1084/jem.20110242
Loh C-Y, Arya A, Naema AF et al (2019) Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: functions and therapeutic implication. Front Oncol 9:48. https://doi.org/10.3389/fonc.2019.00048
Litchfield LM, Appana SN, Datta S, Klinge CM (2014) COUP-TFII inhibits NFkappaB activation in endocrine-resistant breast cancer cells. Mol Cell Endocrinol 382:358–367. https://doi.org/10.1016/j.mce.2013.10.010
Rizk B, Aboulghar M, Smitz J, Ron-El R (1997) The role of vascular endothelial growth factor and interleukins in the pathogenesis of severe ovarian hyperstimulation syndrome. Hum Reprod Update 3:255–266
Lee K-S, Park J-H, Lee S et al (2007) HB-EGF induces delayed STAT3 activation via NF-kappaB mediated IL-6 secretion in vascular smooth muscle cell. Biochim Biophys Acta 1773:1637–1644. https://doi.org/10.1016/j.bbamcr.2007.07.001
Yang M, Wang X, Wang L et al (2017) IL-1α up-regulates IL-6 expression in bovine granulosa cells via MAPKs and NF-κB signaling pathways. Cell Physiol Biochem 41:265–273. https://doi.org/10.1159/000456091
Brasier AR (2010) The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res 86:211–218. https://doi.org/10.1093/cvr/cvq076
Matsuda A, Suzuki Y, Honda G et al (2003) Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene 22:3307–3318. https://doi.org/10.1038/sj.onc.1206406
Van Laere SJ, Van der Auwera I, Van den Eynden GG et al (2007) NF-kappaB activation in inflammatory breast cancer is associated with oestrogen receptor downregulation, secondary to EGFR and/or ErbB2 overexpression and MAPK hyperactivation. Br J Cancer 97:659–669. https://doi.org/10.1038/sj.bjc.6603906
Suh J, Payvandi F, Edelstein LC et al (2002) Mechanisms of constitutive NF-kappaB activation in human prostate cancer cells. Prostate 52:183–200. https://doi.org/10.1002/pros.10082
Zarredar H, Pashapour S, Farajnia S et al (2019) Targeting the KRAS, p38α, and NF-κB in lung adenocarcinoma cancer cells: the effect of combining RNA interferences with a chemical inhibitor. J Cell Biochem. https://doi.org/10.1002/jcb.28357
Li H, Lu S, Chen Y et al (2019) AKT2 phosphorylation of hexokinase 2 at T473 promotes tumorigenesis and metastasis in colon cancer cells via NF-κB, HIF1α, MMP2, and MMP9 upregulation. Cell Signal 58:99–110. https://doi.org/10.1016/j.cellsig.2019.03.011
Koh SY, Moon JY, Unno T, Cho SK (2019) Baicalein suppresses stem cell-like characteristics in radio- and chemoresistant MDA-MB-231 human breast cancer cells through up-regulation of IFIT2. Nutrients. https://doi.org/10.3390/nu11030624
Liang L, Liu X, He J et al (2019) Cyanidin-3-glucoside induces mesenchymal to epithelial transition via activating Sirt1 expression in triple negative breast cancer cells. Biochimie. https://doi.org/10.1016/j.biochi.2019.03.004
Sui H, Lou A, Li Z, Yang J (2019) Lidocaine inhibits growth, migration and invasion of gastric carcinoma cells by up-regulation of miR-145. BMC Cancer 19:233. https://doi.org/10.1186/s12885-019-5431-9
Chen L, Li Q, Zheng Z et al (2019) Design and optimize N-substituted EF24 as effective and low toxicity NF-κB inhibitor for lung cancer therapy via apoptosis-to-pyroptosis switch. Chem Biol Drug Des. https://doi.org/10.1111/cbdd.13514
Page A, Ortega A, Alameda JP et al (2019) IKKα promotes the progression and metastasis of non-small cell lung cancer independently of its subcellular localization. Comput Struct Biotechnol J 17:251–262. https://doi.org/10.1016/j.csbj.2019.02.003
Peng P, Chen T, Wang Q et al (2019) Decreased miR-218-5p levels as a serum biomarker in bone metastasis of prostate cancer. Oncol Res Treat 42:165–185. https://doi.org/10.1159/000495473
Schillaci O, Scimeca M, Trivigno D et al (2019) Prostate cancer and inflammation: a new molecular imaging challenge in the era of personalized medicine. Nucl Med Biol 68–69:66–79. https://doi.org/10.1016/j.nucmedbio.2019.01.003
Hagoel L, Vexler A, Kalich-Philosoph L et al (2019) Combined effect of Moringa oleifera and ionizing radiation on survival and metastatic activity of pancreatic cancer cells. Integr Cancer Ther 18:1534735419828829. https://doi.org/10.1177/1534735419828829
Zhou J, Li Y, Li D et al (2019) Oncoprotein LAMTOR5 activates GLUT1 via upregulating NF-κB in liver cancer. Open Med Wars Pol 14:264–270. https://doi.org/10.1515/med-2019-0022
Jiang Y-W, Cheng H-Y, Kuo C-L et al (2019) Tetrandrine inhibits human brain glioblastoma multiforme GBM 8401 cancer cell migration and invasion in vitro. Environ Toxicol 34:364–374. https://doi.org/10.1002/tox.22691
Lambrou GI, Vlahopoulos S, Papathanasiou C et al (2009) Prednisolone exerts late mitogenic and biphasic effects on resistant acute lymphoblastic leukemia cells: relation to early gene expression. Leuk Res 33:1684–1695. https://doi.org/10.1016/j.leukres.2009.04.018
Lambrou GI, Papadimitriou L, Chrousos GP, Vlahopoulos SA (2012) Glucocorticoid and proteasome inhibitor impact on the leukemic lymphoblast: multiple, diverse signals converging on a few key downstream regulators. Mol Cell Endocrinol 351:142–151. https://doi.org/10.1016/j.mce.2012.01.003
Xu Y, Hu J, Wang X et al (2015) Overexpression of MALT1-A20-NF-κB in adult B-cell acute lymphoblastic leukemia. Cancer Cell Int 15:73. https://doi.org/10.1186/s12935-015-0222-0
Zhu X, Song Y, Wu C et al (2016) Cyr61 participates in the pathogenesis of acute lymphoblastic leukemia by enhancing cellular survival via the AKT/NF-κB signaling pathway. Sci Rep 6:34018. https://doi.org/10.1038/srep34018
Ouimet M, Drouin S, Lajoie M et al (2017) A childhood acute lymphoblastic leukemia-specific lncRNA implicated in prednisolone resistance, cell proliferation, and migration. Oncotarget 8:7477–7488. https://doi.org/10.18632/oncotarget.13936
Ding Y, Gao H, Zhang Q (2017) The biomarkers of leukemia stem cells in acute myeloid leukemia. Stem Cell Investig 4:19. https://doi.org/10.21037/sci.2017.02.10
Goyama S, Mulloy JC (2013) NF-κB: a coordinator for epigenetic regulation by MLL. Cancer Cell 24:401–402. https://doi.org/10.1016/j.ccr.2013.09.016
Zhou X, Li Z, Zhou J (2017) Tumor necrosis factor α in the onset and progression of leukemia. Exp Hematol 45:17–26. https://doi.org/10.1016/j.exphem.2016.10.005
Lambrou GI, Adamaki M, Vlahopoulos S, Hatziagapiou K (2020) Gene expression and resistance to glucocorticoid-induced apoptosis in acute lymphoblastic leukemia: a brief review and update. Curr Drug Res Rev. https://doi.org/10.2174/2589977512666200220122650
Sheng W-Y, Chen Y-R, Wang T-CV (2006) A major role of PKC theta and NFkappaB in the regulation of hTERT in human T lymphocytes. FEBS Lett 580:6819–6824. https://doi.org/10.1016/j.febslet.2006.11.044
Chen H, Li C, Peng X et al (2018) A pan-cancer analysis of enhancer expression in nearly 9000 patient samples. Cell 173:386–399.e12. https://doi.org/10.1016/j.cell.2018.03.027
Gershkovitz M, Fainsod-Levi T, Khawaled S et al (2018) Microenvironmental cues determine tumor cell susceptibility to neutrophil cytotoxicity. Cancer Res 78:5050–5059. https://doi.org/10.1158/0008-5472.CAN-18-0540
Gershkovitz M, Fainsod-Levi T, Zelter T et al (2019) TRPM2 modulates neutrophil attraction to murine tumor cells by regulating CXCL2 expression. Cancer Immunol Immunother 68:33–43. https://doi.org/10.1007/s00262-018-2249-2
Jablonska J, Lang S, Sionov RV, Granot Z (2017) The regulation of pre-metastatic niche formation by neutrophils. Oncotarget 8:112132–112144. https://doi.org/10.18632/oncotarget.22792
Martincuks A, Andryka K, Küster A et al (2017) Nuclear translocation of STAT3 and NF-κB are independent of each other but NF-κB supports expression and activation of STAT3. Cell Signal 32:36–47. https://doi.org/10.1016/j.cellsig.2017.01.006
Kosack L, Wingelhofer B, Popa A et al (2019) The ERBB-STAT3 axis drives Tasmanian devil facial tumor disease. Cancer Cell 35:125–139.e9. https://doi.org/10.1016/j.ccell.2018.11.018
Adamaki M, Tsotra M, Vlahopoulos S et al (2015) STAT transcript levels in childhood acute lymphoblastic leukemia: STAT1 and STAT3 transcript correlations. Leuk Res. https://doi.org/10.1016/j.leukres.2015.09.004
Dmitrieva OS, Shilovskiy IP, Khaitov MR, Grivennikov SI (2016) Interleukins 1 and 6 as main mediators of inflammation and cancer. Biochem Biokhimiia 81:80–90. https://doi.org/10.1134/S0006297916020024
Goldstein I, Paakinaho V, Baek S et al (2017) Synergistic gene expression during the acute phase response is characterized by transcription factor assisted loading. Nat Commun 8:1849. https://doi.org/10.1038/s41467-017-02055-5
Campbell KJ, Bath ML, Turner ML et al (2010) Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood 116:3197–3207. https://doi.org/10.1182/blood-2010-04-281071
Nakaya A, Sagawa M, Muto A et al (2011) The gold compound auranofin induces apoptosis of human multiple myeloma cells through both down-regulation of STAT3 and inhibition of NF-κB activity. Leuk Res 35:243–249. https://doi.org/10.1016/j.leukres.2010.05.011
Argyrou C, Hatziagapiou K, Theodorakidou M et al (2019) The role of adiponectin, LEPTIN, and ghrelin in the progress and prognosis of childhood acute lymphoblastic leukemia. Leuk Lymphoma. https://doi.org/10.1080/10428194.2019.156923
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
GIL: Reviewed literature, drafted manuscript, reviewed manuscript, proof-edited the manuscript. KH: drafted the manuscript, proof-read the manuscript. SV: edited the manuscript, proof-read the manuscript and gave final permission for publication.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lambrou, G.I., Hatziagapiou, K. & Vlahopoulos, S. Inflammation and tissue homeostasis: the NF-κB system in physiology and malignant progression. Mol Biol Rep 47, 4047–4063 (2020). https://doi.org/10.1007/s11033-020-05410-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05410-w