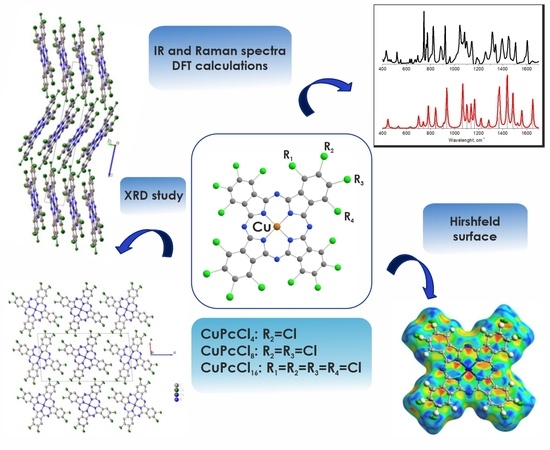
Chlorosubstituted Copper Phthalocyanines: Spectral Study and Structure of Thin Films
Abstract
:1. Introduction
2. Results and Discussion
2.1. Single Crystal Structure of CuPcCl4
2.2. Vibrational Spectra
2.3. XRD Study of CuPcCl4, CuPcCl8 and CuPcCl16 Thin Films
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shigemitsu, M. Syntheses of Chlorinated Copper Phthalocyanines from Chlorophthalic Anhydrides. Bull. Chem. Soc. Jpn. 1959, 32, 691–693. [Google Scholar]
- Abe, T.; Nagai, K. Novel photofunctions of bilayer composed of p-type phthalocyanine and n-type organic semiconductor as photoelectrodes in the water phase. Org. Electron. 2007, 8, 262–271. [Google Scholar]
- Nénon, S.; Kanehira, D.; Yoshimoto, N.; Fages, F.; Videlot-Ackermann, C. Shelf-life time test of p- and n-channel organic thin film transistors using copper phthalocyanines. Thin Solid Films 2010, 518, 5593–5598. [Google Scholar]
- Bao, Z.; Lovinger, A.J.; Brown, J. New Air-Stable n-Channel Organic Thin Film Transistors. J. Am. Chem. Soc. 1998, 120, 207–208. [Google Scholar]
- Ma, F.; Wang, S.; Li, X. Synthesis, spectral characterization of CuPcF16 and its application in organic thin film transistors using p-6p as inducing layer. J. Phys. Chem. Solids 2012, 73, 589–592. [Google Scholar]
- Shao, X.; Wang, S.; Li, X.; Su, Z.; Chen, Y.; Xiao, Y. Single component p-, ambipolar and n-type OTFTs based on fluorinated copper phthalocyanines. Dye. Pigment. 2016, 132, 378–386. [Google Scholar]
- Wu, F.-C.; Cheng, H.-L.; Yen, C.-H.; Lin, J.-W.; Liu, S.-J.; Chou, W.-Y.; Tang, F.-C. Electron transport properties in fluorinated copper–phthalocyanine films: Importance of vibrational reorganization energy and molecular microstructure. Phys. Chem. Chem. Phys. 2010, 12, 2098. [Google Scholar]
- Oh, Y.; Pyo, S.; Yi, M.; Kwon, S.-K. N-type organic field-effect transistor using polymeric blend gate insulator with controlled surface properties. Org. Electron. 2006, 7, 77–84. [Google Scholar]
- Klyamer, D.D.; Sukhikh, A.S.; Krasnov, P.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V. Thin films of tetrafluorosubstituted cobalt phthalocyanine: Structure and sensor properties. Appl. Surf. Sci. 2016, 372, 79–86. [Google Scholar]
- Parkhomenko, R.; Sukhikh, A.S.; Klyamer, D.D.; Krasnov, P.; Gromilov, S.; Kadem, B.; Hassan, A.K.; Basova, T.V. Thin Films of Unsubstituted and Fluorinated Palladium Phthalocyanines: Structure and Sensor Response toward Ammonia and Hydrogen. J. Phys. Chem. C 2017, 121, 1200–1209. [Google Scholar]
- Klyamer, D.D.; Sukhikh, A.S.; Trubin, S.V.; Gromilov, S.A.; Morozova, N.B.; Basova, T.V.; Hassan, A.K. Tetrafluorosubstituted Metal Phthalocyanines: Interplay between Saturated Vapor Pressure and Crystal Structure. Cryst. Growth Des. 2020, 20, 1016–1024. [Google Scholar] [CrossRef]
- Sharma, R.K.; Gulati, S.; Sachdeva, S. One pot and solvent-free synthesis of 2,9,16,23-tetrachlorometal(II) phthalocyanines. Green Chem. Lett. Rev. 2012, 5, 83–87. [Google Scholar] [CrossRef]
- Safari, N.; Jamaat, P.R.; Pirouzmand, M.; Shaabani, A. Synthesis of metallophthalocyanines using microwave irradiation under solvent free and reflux conditions. J. Porphyrins Phthalocyanines 2004, 8, 1209–1213. [Google Scholar] [CrossRef]
- Safari, N.; Jamaat, P.R.; Shirvan, S.A.; Shoghpour, S.; Ebadi, A.; Darvishi, M.; Shaabani, A. Rapid and efficient synthesis of metallophthalocyanines in ionic liquid. J. Porphyrins Phthalocyanines 2005, 9, 256–261. [Google Scholar] [CrossRef]
- Lomova, T.N.; Sokolova, T.N.; Zaitseva, S.; Zdanovich, S.; Maizlish, V.E. Structure and properties of tetrakis[3(4)-chlorophthalocyaninato]copper(II) protonated forms in the isolated state and in the sulfuric acid solutions. Russ. J. Gen. Chem. 2013, 83, 1563–1570. [Google Scholar] [CrossRef]
- Zięba-Palus, J.; Michalska, A. Characterization of Blue Pigments Used in Automotive Paints by Raman Spectroscopy. J. Forensic Sci. 2014, 59, 943–949. [Google Scholar] [CrossRef]
- Duce, C.; Della Porta, V.; Tiné, M.R.; Spepi, A.; Ghezzi, L.; Colombini, M.P.; Bramanti, E. FTIR study of ageing of fast drying oil colour (FDOC) alkyd paint replicas. Spectrochim. Acta Part. A: Mol. Biomol. Spectrosc. 2014, 130, 214–221. [Google Scholar] [CrossRef]
- Pakhomov, L.G.; Pakhomov, G.L. NO2 interaction with thin film of phthalocyanine derivatives {1}. Synth. Met. 1995, 71, 2299–2300. [Google Scholar] [CrossRef]
- Irie, S.; Hoshino, A.; Kuwamoto, K.; Isoda, S.; Miles, M.; Kobayashi, T. Point-on-line coincidence in epitaxial growth of CuPcCl16 on graphite. Appl. Surf. Sci. 1997, 113, 310–315. [Google Scholar] [CrossRef]
- Mittelberger, A.; Kramberger, C.; Meyer, J.C. Insights into radiation damage from atomic resolution scanning transmission electron microscopy imaging of mono-layer CuPcCl16 films on graphene. Sci. Rep. 2018, 8, 4813. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Biskupek, J.; Kurata, H.; Kaiser, U. Critical conditions for atomic resolution imaging of molecular crystals by aberration-corrected HRTEM. Ultramicroscopy 2015, 159, 73–80. [Google Scholar] [CrossRef]
- Bobaru, S.C.; Salomon, E.; Layet, J.-M.; Angot, T. Structural Properties of Iron Phtalocyanines on Ag(111): From the Submonolayer to Monolayer Range. J. Phys. Chem. C 2011, 115, 5875–5879. [Google Scholar] [CrossRef]
- Amsalem, P.; Giovanelli, L.; Themlin, J.M.; Koudia, M.; Abel, M.; Oison, V.; Ksari, Y.; Mossoyan, M.; Porte, L. Interface formation and growth of a thin film of ZnPcCl8/Ag(111) studied by photoelectron spectroscopy. Surf. Sci. 2007, 601, 4185–4188. [Google Scholar] [CrossRef]
- Haruta, M.; Kurata, H. Direct observation of crystal defects in an organic molecular crystals of copper hexachlorophthalocyanine by STEM-EELS. Sci. Rep. 2012, 2, 252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fryer, J.R. Electron Crystallography of Phthalocyanines. J. Porphyr. Phthalocyanine 1999, 3, 672–678. [Google Scholar] [CrossRef]
- Selvaraj, T.; Rajalingam, R. Theoretical Studies of the Zeolite-Y Encapsulated Chlorine-Substituted Copper(II)phthalocyanine Complex on the Formation Glycidol from Allyl Alcohol. ACS Omega 2018, 3, 9613–9619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.R.; Sahu, S.; Sharma, S. Charge transport and prototypical optical absorptions in functionalized zinc phthalocyanine compounds: A density functional study. J. Phys. Org. Chem. 2017, 31, e3785. [Google Scholar] [CrossRef]
- Koshy, R.; Menon, C.S. Influence of air annealing and gamma ray irradiation on the optical properties of Cl16FePc thin films. E-Journal Chem. 2012, 9, 2439–2445. [Google Scholar] [CrossRef]
- Achar, B.N.; Jayasree, P.K. “Molecular Metals” Based on Copper(II) 2,9,16,23-tetrahalo Substituted Phthalocyanine Derivatives. Synth. React. Inorg. Met. Chem. 2000, 30, 719–733. [Google Scholar] [CrossRef]
- Ling, M.-M.; Bao, Z.; Erk, P. Air-stable n-channel copper hexachlorophthalocyanine for field-effect transistors. Appl. Phys. Lett. 2006, 89, 163516. [Google Scholar] [CrossRef]
- Honigmann, B.; Lenne, H.U.; Schrödel, R.; Anilin, B. Beziehungen zwischen den strukturen der modifikationen des platin- und kupferphthalocyanins und einiger chlorderivate. Zeitschrift fur Krist. - New Cryst. Struct. 1965, 122, 185–205. [Google Scholar]
- Brown, C.J. Crystal structure of β-copper phthalocyanine. J. Chem. Soc. A 1968, 2488. [Google Scholar] [CrossRef]
- Erk, P.; Hengelsberg, H.; Haddow, M.; Van Gelder, R. The innovative momentum of crystal engineering. CrystEngComm 2004, 6, 474. [Google Scholar] [CrossRef]
- Basova, T.V.; Kiselev, V.; Schuster, B.-E.; Peisert, H.; Chassé, T. Experimental and theoretical investigation of vibrational spectra of copper phthalocyanine: Polarized single-crystal Raman spectra, isotope effect and DFT calculations. J. Raman Spectrosc. 2009, 40, 2080–2087. [Google Scholar] [CrossRef]
- Uyeda, N. Molecular image resolution in electron microscopy. J. Appl. Phys. 1972, 43, 5181. [Google Scholar] [CrossRef]
- Rüffer, T.; Nurpeisova, D.; Jakupova, Z.; Tashenov, A.; Uhlig, N.; Khalladi, A.; Mertens, L.; Gonser, A.; Mehring, M.; Lang, H. Synthesis and purification of metallooctachlorophthalocyanines. Zeitschrift für Naturforschung B 2017, 72, 589–601. [Google Scholar] [CrossRef]
- Louër, D.; Boultif, A. Some further considerations in powder diffraction pattern indexing with the dichotomy method. Powder Diffr. 2014, 29, S7–S12. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, P.; Ye, J.; Li, Y.; Li, H.; Zhang, X.; Li, R.; Dong, H.; Hu, W.; Kloc, C. Molecular Crystal Engineering: Tuning Organic Semiconductor from p-type to n-type by Adjusting Their Substitutional Symmetry. Adv. Mater. 2017, 29, 1605053. [Google Scholar] [CrossRef]
- Klyamer, D.D.; Sukhikh, A.S.; Gromilov, S.A.; Kruchinin, V.N.; Spesivtsev, E.V.; Hassan, A.K.; Basova, T.V. Influence of Fluorosubstitution on the Structure of Zinc Phthalocyanine Thin Films. Macroheterocycles 2018, 11, 304–311. [Google Scholar] [CrossRef]
- APEX3; v.2018-7.2; Bruker AXS, Inc.: Madison, WI, USA, 2018.
- Dolomanov, O.; Bourhis, L.J.; Gildea, R.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT - integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Jayatilaka, D.; Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Spackman, M.A. CrystalExplorer: A tool for displaying Hirshfeld surfaces and visualising intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 2006, 62, s90. [Google Scholar] [CrossRef]
- Jayatilaka, D.; Grimwood, D. Tonto: A Fortran Based Object-Oriented System for Quantum Chemistry and Crystallography. Computer Vision 2003, 2660, 142–151. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Hehre, W.J. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chem. Accounts 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Rassolov, V.; Windus, T.L.; Pople, J.A.; Ratner, M.A. 6-31G* basis set for atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds of CuPcCl4, CuPcCl8 and CuPcCl16 are available from the authors. |







| Empirical Formula | C32H12Cl4CuN8 | |
|---|---|---|
| Formula weight | 713.84 | |
| Temperature/K | 150 | 298 |
| Crystal system | monoclinic | monoclinic |
| Space group | P21/c | P21/c |
| a/Å | 14.0052(9) | 14.080(4) |
| b/Å | 3.6376(3) | 3.6823(8) |
| c/Å | 26.5123(18) | 26.693(5) |
| α/° | 90 | 90 |
| β/° | 94.893(3) | 94.636(20) |
| γ/° | 90 | 90 |
| Volume/Å3 | 1345.76(17) | 1379.4(5) |
| Z | 2 | 2 |
| ρcalcg/cm3 | 1.762 | 1.806 |
| Reflections collected | 12242 | N/A |
| Independent reflections | 2580 (Rint = 9.05%) | N/A |
| Data/restraints/parameters | 2508/0/225 | N/A |
| Goodness-of-fit on F2 | 1.010 | N/A |
| R indices [I > = 2σ (I)] | R1 = 4.90%, wR2 = 9.02% | N/A |
| R indices [all data] | R1 = 12.03%, wR2 = 11.05% | N/A |
| CCDC № | 1972791 | N/A |
| Bond | CuPcCl4 | CuPcCl8 | CuPcCl16 | |
|---|---|---|---|---|
| Experimental | Calculated | |||
| Cu-Nα | 1.942 | 1.951 | 1.951 | 1.950 |
| Cα-Nα | 1.372 | 1.377 | 1.376 | 1.372 |
| Cα-Nβ | 1.332 | 1.324 | 1.324 | 1.322 |
| Cα-Cβ | 1.454 | 1.456 | 1.457 | 1.465 |
| Cβ-Cβ | 1.392 | 1.405 | 1.404 | 1.412 |
| Cβ-Cγ | 1.391 | 1.396 | 1.392 | 1.400 |
| Cγ-Cδ | 1.376 | 1.392 | 1.394 | 1.404 |
| Cδ-Cδ | 1.393 | 1.408 | 1.418 | 1.415 |
| Cγ-H | n/a | 1.084 | 1.084 | - |
| Cδ-H | n/a | 1.084 | - | - |
| Cγ-Cl | - | - | - | 1.736 |
| Cδ-Cl | 1.682 | 1.758 | 1.746 | 1.732 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhikh, A.; Bonegardt, D.; Klyamer, D.; Krasnov, P.; Basova, T. Chlorosubstituted Copper Phthalocyanines: Spectral Study and Structure of Thin Films. Molecules 2020, 25, 1620. https://doi.org/10.3390/molecules25071620
Sukhikh A, Bonegardt D, Klyamer D, Krasnov P, Basova T. Chlorosubstituted Copper Phthalocyanines: Spectral Study and Structure of Thin Films. Molecules. 2020; 25(7):1620. https://doi.org/10.3390/molecules25071620
Chicago/Turabian StyleSukhikh, Alexandr, Dmitry Bonegardt, Darya Klyamer, Pavel Krasnov, and Tamara Basova. 2020. "Chlorosubstituted Copper Phthalocyanines: Spectral Study and Structure of Thin Films" Molecules 25, no. 7: 1620. https://doi.org/10.3390/molecules25071620