Abstract
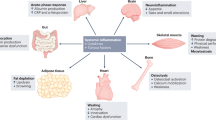
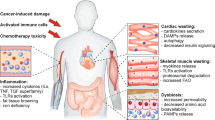
Tumours reprogram host physiology, metabolism and immune responses during cancer progression. The release of soluble factors, exosomes and metabolites from tumours leads to systemic changes in distant organs, where cancer cells metastasize and grow. These tumour-derived circulating factors also profoundly impact tissues that are rarely inhabited by metastatic cancer cells such as skeletal muscle and adipose tissue. In fact, the majority of patients with metastatic cancer develop a debilitating muscle-wasting syndrome, known as cachexia, that is associated with decreased tolerance to antineoplastic therapy, poor prognosis and accelerated death, with no approved treatments. In this Perspective, we discuss the development of cachexia in the context of metastatic progression. We briefly discuss how circulating factors either directly or indirectly promote cachexia development and examine how signals from the metastatic process can trigger and amplify this process. Finally, we highlight promising therapeutic opportunities for targeting cachexia in the context of metastatic cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Fearon, K. C., Glass, D. J. & Guttridge, D. C. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 16, 153–166 (2012).
Fearon, K., Arends, J. & Baracos, V. Understanding the mechanisms and treatment options in cancer cachexia. Nat. Rev. Clin. Oncol. 10, 90–99 (2013).
Bruera, E. & Sweeney, C. Cachexia and asthenia in cancer patients. Lancet Oncol. 1, 138–147 (2000).
Kalantar-Zadeh, K. et al. Why cachexia kills: examining the causality of poor outcomes in wasting conditions. J. Cachexia Sarcopenia Muscle 4, 89–94 (2013).
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 4, 17105 (2018).
Ni, X., Yang, J. & Li, M. Imaging-guided curative surgical resection of pancreatic cancer in a xenograft mouse model. Cancer Lett. 324, 179–185 (2012).
Gallagher, I. J. et al. Suppression of skeletal muscle turnover in cancer cachexia: evidence from the transcriptome in sequential human muscle biopsies. Clin. Cancer Res. 18, 2817–2827 (2012).
Salazar-Degracia, A. et al. Reduced lung cancer burden by selective immunomodulators elicits improvements in muscle proteolysis and strength in cachectic mice. J. Cell Physiol. 234, 18041–18052 (2019).
Wang, G. et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat. Med. 24, 770–781 (2018).
Anderson, R. L. et al. A framework for the development of effective anti-metastatic agents. Nat. Rev. Clin. Oncol. 16, 185–204 (2019).
Jatoi, A. et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 68, 234–239 (2010).
Penna, F., Busquets, S. & Argiles, J. M. Experimental cancer cachexia: evolving strategies for getting closer to the human scenario. Semin. Cell Dev. Biol. 54, 20–27 (2016).
Barreto, R. et al. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 7, 43442–43460 (2016).
Gilliam, L. A., Moylan, J. S., Callahan, L. A., Sumandea, M. P. & Reid, M. B. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve 43, 94–102 (2011).
Damrauer, J. S. et al. Chemotherapy-induced muscle wasting: association with NF-κB and cancer cachexia. Eur. J. Transl. Myol. 28, 7590 (2018).
Waning, D. L. et al. Excess TGF-β mediates muscle weakness associated with bone metastases in mice. Nat. Med. 21, 1262–1271 (2015).
Greco, S. H. et al. TGF-β blockade reduces mortality and metabolic changes in a validated murine model of pancreatic cancer cachexia. PLoS One 10, e0132786 (2015).
Go, K. L. et al. Orthotopic patient-derived pancreatic cancer xenografts engraft into the pancreatic parenchyma, metastasize, and induce muscle wasting to recapitulate the human disease. Pancreas 46, 813–819 (2017).
Norton, J. A., Moley, J. F., Green, M. V., Carson, R. E. & Morrison, S. D. Parabiotic transfer of cancer anorexia/cachexia in male rats. Cancer Res. 45, 5547–5552 (1985).
Argiles, J. M., Stemmler, B., Lopez-Soriano, F. J. & Busquets, S. Nonmuscle tissues contribution to cancer cachexia. Mediators Inflamm. 2015, 182872 (2015).
Argiles, J. M., Lopez-Soriano, F. J. & Busquets, S. Mediators of cachexia in cancer patients. Nutrition 66, 11–15 (2019).
Petruzzelli, M. & Wagner, E. F. Mechanisms of metabolic dysfunction in cancer-associated cachexia. Genes. Dev. 30, 489–501 (2016).
Sandri, M. Protein breakdown in cancer cachexia. Semin. Cell Dev. Biol. 54, 11–19 (2016).
Baracos, V. E., DeVivo, C., Hoyle, D. H. & Goldberg, A. L. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am. J. Physiol. 268, E996–E1006 (1995).
Stitt, T. N. et al. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell 14, 395–403 (2004).
Sandri, M. et al. FOXO transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 (2004).
Honors, M. A. & Kinzig, K. P. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J. Cachexia Sarcopenia Muscle 3, 5–11 (2012).
Heber, D., Byerly, L. O. & Chlebowski, R. T. Metabolic abnormalities in the cancer patient. Cancer 55, 225–229 (1985).
Cahill, G. F. Jr, Aoki, T. T., Brennan, M. F. & Muller, W. A. Insulin and muscle amino acid balance. Proc. Nutr. Soc. 31, 233–238 (1972).
Voet, D. & Voet, J. G. Biochemistry. 4th edn (Wiley, 2011).
Wang, X., Hu, Z., Hu, J., Du, J. & Mitch, W. E. Insulin resistance accelerates muscle protein degradation: Activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147, 4160–4168 (2006).
Fernandes, L. C., Machado, U. F., Nogueira, C. R., Carpinelli, A. R. & Curi, R. Insulin secretion in Walker 256 tumor cachexia. Am. J. Physiol. 258, E1033–E1036 (1990).
Asp, M. L., Tian, M., Wendel, A. A. & Belury, M. A. Evidence for the contribution of insulin resistance to the development of cachexia in tumor-bearing mice. Int. J. Cancer 126, 756–763 (2010).
Kwon, Y. et al. Systemic organ wasting induced by localized expression of the secreted insulin/IGF antagonist ImpL2. Dev. Cell 33, 36–46 (2015).
Figueroa-Clarevega, A. & Bilder, D. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev. Cell 33, 47–55 (2015).
Asp, M. L., Tian, M., Kliewer, K. L. & Belury, M. A. Rosiglitazone delayed weight loss and anorexia while attenuating adipose depletion in mice with cancer cachexia. Cancer Biol. Ther. 12, 957–965 (2011).
Trobec, K. et al. Rosiglitazone reduces body wasting and improves survival in a rat model of cancer cachexia. Nutrition 30, 1069–1075 (2014).
Bhatnagar, S., Mittal, A., Gupta, S. K. & Kumar, A. TWEAK causes myotube atrophy through coordinated activation of ubiquitin-proteasome system, autophagy, and caspases. J. Cell Physiol. 227, 1042–1051 (2012).
Cai, D. et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119, 285–298 (2004).
Guttridge, D. C., Mayo, M. W., Madrid, L. V., Wang, C. Y. & Baldwin, A. S. Jr. NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289, 2363–2366 (2000).
Fukawa, T. et al. Excessive fatty acid oxidation induces muscle atrophy in cancer cachexia. Nat. Med. 22, 666–671 (2016).
Zimmers, T. A. et al. Induction of cachexia in mice by systemically administered myostatin. Science 296, 1486–1488 (2002).
Benny Klimek, M. E. et al. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem. Biophys. Res. Commun. 391, 1548–1554 (2010).
Zhou, X. et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142, 531–543 (2010).
Yang, L. et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 23, 1158–1166 (2017).
Emmerson, P. J. et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 23, 1215–1219 (2017).
Hsu, J. Y. et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 550, 255–259 (2017).
Tsai, V. W. et al. TGF-β superfamily cytokine MIC-1/GDF15 is a physiological appetite and body weight regulator. PLoS One 8, e55174 (2013).
Johnen, H. et al. Tumor-induced anorexia and weight loss are mediated by the TGF-β superfamily cytokine MIC-1. Nat. Med. 13, 1333–1340 (2007).
Hammers, D. W. et al. Supraphysiological levels of GDF11 induce striated muscle atrophy. EMBO Mol. Med. 9, 531–544 (2017).
Zimmers, T. A. et al. Exogenous GDF11 induces cardiac and skeletal muscle dysfunction and wasting. Basic. Res. Cardiol. 112, 48 (2017).
Jones, J. E. et al. Supraphysiologic administration of GDF11 induces cachexia in part by upregulating GDF15. Cell Rep. 22, 1522–1530 (2018).
Wortzel, I., Dror, S., Kenific, C. M. & Lyden, D. Exosome-mediated metastasis: communication from a distance. Dev. Cell 49, 347–360 (2019).
Zhang, G. et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun. 8, 589 (2017).
Yang, J. et al. ZIP4 promotes muscle wasting and cachexia in mice with orthotopic pancreatic tumors by stimulating RAB27B-regulated release of extracellular vesicles from cancer cells. Gastroenterology 156, 722–734 (2019).
He, W. A. et al. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl Acad. Sci. USA 111, 4525–4529 (2014).
Calore, F. et al. The TLR7/8/9 antagonist IMO-8503 inhibits cancer-induced cachexia. Cancer Res. 78, 6680–6690 (2018).
Das, S. K. et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333, 233–238 (2011).
Giralt, M. & Villarroya, F. White, brown, beige/brite: different adipose cells for different functions? Endocrinology 154, 2992–3000 (2013).
Kliewer, K. L. et al. Adipose tissue lipolysis and energy metabolism in early cancer cachexia in mice. Cancer Biol. Ther. 16, 886–897 (2015).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Kir, S. et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 513, 100–104 (2014).
Ang, Q. Y. et al. A new method of infrared thermography for quantification of brown adipose tissue activation in healthy adults (TACTICAL): a randomized trial. J. Physiol. Sci. 67, 395–406 (2017).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Mueller-Klieser, W., Walenta, S., Paschen, W., Kallinowski, F. & Vaupel, P. Metabolic imaging in microregions of tumors and normal tissues with bioluminescence and photon counting. J. Natl Cancer Inst. 80, 842–848 (1988).
Walenta, S. et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 60, 916–921 (2000).
Friesen, D. E., Baracos, V. E. & Tuszynski, J. A. Modeling the energetic cost of cancer as a result of altered energy metabolism: implications for cachexia. Theor. Biol. Med. Model. 12, 17 (2015).
Argiles, J. M., Fontes-Oliveira, C. C., Toledo, M., Lopez-Soriano, F. J. & Busquets, S. Cachexia: a problem of energetic inefficiency. J. Cachexia Sarcopenia Muscle 5, 279–286 (2014).
Felig, P., Pozefsky, T., Marliss, E. & Cahill, G. F. Jr. Alanine: key role in gluconeogenesis. Science 167, 1003–1004 (1970).
Ishikawa, E. The regulation of uptake and output of amino acids by rat tissues. Adv. Enzyme Regul. 14, 117–136 (1976).
Felig, P. & Wahren, J. Amino acid metabolism in exercising man. J. Clin. Invest. 50, 2703–2714 (1971).
Stephens, N. A., Skipworth, R. J. & Fearon, K. C. Cachexia, survival and the acute phase response. Curr. Opin. Support. Palliat. Care 2, 267–274 (2008).
Bonetto, A. et al. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS One 6, e22538 (2011).
Kushner, I. The phenomenon of the acute phase response. Ann. NY Acad. Sci. 389, 39–48 (1982).
Reeds, P. J., Fjeld, C. R. & Jahoor, F. Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J. Nutr. 124, 906–910 (1994).
Preston, T. et al. Fibrinogen synthesis is elevated in fasting cancer patients with an acute phase response. J. Nutr. 128, 1355–1360 (1998).
Goncalves, M. D. et al. Fenofibrate prevents skeletal muscle loss in mice with lung cancer. Proc. Natl Acad. Sci. USA 115, E743–E752 (2018).
Flint, T. R. et al. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 24, 672–684 (2016).
Schakman, O., Gilson, H. & Thissen, J. P. Mechanisms of glucocorticoid-induced myopathy. J. Endocrinol. 197, 1–10 (2008).
Clarke, B. A. et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 6, 376–385 (2007).
Braun, T. P. et al. Cancer- and endotoxin-induced cachexia require intact glucocorticoid signaling in skeletal muscle. FASEB J. 27, 3572–3582 (2013).
Huot, J. R., Novinger, L. J., Pin, F. & Bonetto, A. HCT116 colorectal liver metastases exacerbate muscle wasting in a mouse model for the study of colorectal cancer cachexia. Dis. Model. Mech. https://doi.org/10.1242/dmm.043166 (2020).
Lauffenburger, D. A. & Horwitz, A. F. Cell migration: a physically integrated molecular process. Cell 84, 359–369 (1996).
Dongre, A. & Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 (2019).
Rasanen, K. et al. Comparative secretome analysis of epithelial and mesenchymal subpopulations of head and neck squamous cell carcinoma identifies S100A4 as a potential therapeutic target. Mol. Cell Proteom. 12, 3778–3792 (2013).
Suarez-Carmona, M. et al. Soluble factors regulated by epithelial-mesenchymal transition mediate tumour angiogenesis and myeloid cell recruitment. J. Pathol. 236, 491–504 (2015).
Fernando, R. I., Castillo, M. D., Litzinger, M., Hamilton, D. H. & Palena, C. IL-8 signaling plays a critical role in the epithelial-mesenchymal transition of human carcinoma cells. Cancer Res. 71, 5296–5306 (2011).
Lu, H. et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 16, 1105–1117 (2014).
Hartman, Z. C. et al. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res. 73, 3470–3480 (2013).
Ginestier, C. et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Invest. 120, 485–497 (2010).
Strassmann, G., Fong, M., Kenney, J. S. & Jacob, C. O. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J. Clin. Invest. 89, 1681–1684 (1992).
Ohe, Y. et al. Interleukin-6 cDNA transfected Lewis lung carcinoma cells show unaltered net tumour growth rate but cause weight loss and shortened survival in syngeneic mice. Br. J. Cancer 67, 939–944 (1993).
Callaway, C. S. et al. IL-8 released from human pancreatic cancer and tumor-associated stromal cells signals through a CXCR2-ERK1/2 axis to induce muscle atrophy. Cancers 11, E1863 (2019).
Kaplan, R. N. et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827 (2005).
Peinado, H. et al. Pre-metastatic niches: organ-specific homes for metastases. Nat. Rev. Cancer 17, 302–317 (2017).
Costa-Silva, B. et al. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826 (2015).
Hiratsuka, S., Watanabe, A., Aburatani, H. & Maru, Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375 (2006).
Hiratsuka, S. et al. Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nat. Commun. 4, 1853 (2013).
Kim, S. et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 457, 102–106 (2009).
Lee, J. W. et al. Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature 567, 249–252 (2019).
Hiratsuka, S. et al. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nat. Cell Biol. 10, 1349–1355 (2008).
Kowanetz, M. et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc. Natl Acad. Sci. USA 107, 21248–21255 (2010).
Lambert, A. W., Pattabiraman, D. R. & Weinberg, R. A. Emerging biological principles of metastasis. Cell 168, 670–691 (2017).
Esposito, M., Guise, T. & Kang, Y. The biology of bone metastasis. Cold Spring Harb. Perspect. Med. 8, a031252 (2018).
Mundy, G. R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 (2002).
Yin, J. J. et al. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 103, 197–206 (1999).
Wang, W. et al. Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene 38, 4540–4559 (2019).
Kalli, M. et al. Solid stress-induced migration is mediated by GDF15 through Akt pathway activation in pancreatic cancer cells. Sci. Rep. 9, 978 (2019).
Bruzzese, F. et al. Local and systemic protumorigenic effects of cancer-associated fibroblast-derived GDF15. Cancer Res. 74, 3408–3417 (2014).
Li, C. et al. GDF15 promotes EMT and metastasis in colorectal cancer. Oncotarget 7, 860–872 (2016).
Brown, D. A. et al. MIC-1 serum level and genotype: associations with progress and prognosis of colorectal carcinoma. Clin. Cancer Res. 9, 2642–2650 (2003).
Lerner, L. et al. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J. Cachexia Sarcopenia Muscle 6, 317–324 (2015).
Tsai, V. W., Brown, D. A. & Breit, S. N. Targeting the divergent TGFβ superfamily cytokine MIC-1/GDF15 for therapy of anorexia/cachexia syndromes. Curr. Opin. Support. Palliat. Care 12, 404–409 (2018).
Zugmaier, G. et al. Transforming growth factor beta 1 induces cachexia and systemic fibrosis without an antitumor effect in nude mice. Cancer Res. 51, 3590–3594 (1991).
Iguchi, H., Onuma, E., Sato, K., Sato, K. & Ogata, E. Involvement of parathyroid hormone-related protein in experimental cachexia induced by a human lung cancer-derived cell line established from a bone metastasis specimen. Int. J. Cancer 94, 24–27 (2001).
Stewart, A. F. Clinical practice. Hypercalcemia associated with cancer. N. Engl. J. Med. 352, 373–379 (2005).
Guillen, C., Martinez, P., de Gortazar, A. R., Martinez, M. E. & Esbrit, P. Both N- and C-terminal domains of parathyroid hormone-related protein increase interleukin-6 by nuclear factor-κB activation in osteoblastic cells. J. Biol. Chem. 277, 28109–28117 (2002).
Pollock, J. H., Blaha, M. J., Lavish, S. A., Stevenson, S. & Greenfield, E. M. In vivo demonstration that parathyroid hormone and parathyroid hormone-related protein stimulate expression by osteoblasts of interleukin-6 and leukemia inhibitory factor. J. Bone Min. Res. 11, 754–759 (1996).
Seto, D. N., Kandarian, S. C. & Jackman, R. W. A key role for leukemia inhibitory factor in C26 cancer cachexia. J. Biol. Chem. 290, 19976–19986 (2015).
Sakai, R. & Eto, Y. Involvement of activin in the regulation of bone metabolism. Mol. Cell Endocrinol. 180, 183–188 (2001).
Leto, G. et al. Activin A circulating levels in patients with bone metastasis from breast or prostate cancer. Clin. Exp. Metastasis 23, 117–122 (2006).
Chen, J. L. et al. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 28, 1711–1723 (2014).
Lee, S. J. et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc. Natl Acad. Sci. USA 102, 18117–18122 (2005).
Matzuk, M. M. et al. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc. Natl Acad. Sci. USA 91, 8817–8821 (1994).
Morvan, F. et al. Blockade of activin type II receptors with a dual anti-ActRIIA/IIB antibody is critical to promote maximal skeletal muscle hypertrophy. Proc. Natl Acad. Sci. USA 114, 12448–12453 (2017).
Obenauf, A. C. & Massague, J. Surviving at a distance: organ specific metastasis. Trends Cancer 1, 76–91 (2015).
Shakri, A. R. et al. Upregulation of ZIP14 and altered zinc homeostasis in muscles in pancreatic cancer cachexia. Cancers 12, E3 (2019).
Gupta, S. K., Shukla, V. K., Vaidya, M. P., Roy, S. K. & Gupta, S. Serum and tissue trace elements in colorectal cancer. J. Surg. Oncol. 52, 172–175 (1993).
Morikawa, K., Walker, S. M., Jessup, J. M. & Fidler, I. J. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 48, 1943–1948 (1988).
Tseng, Y. C. et al. Preclinical investigation of the novel histone deacetylase inhibitor AR-42 in the treatment of cancer-induced cachexia. J. Natl Cancer Inst. 107, djv274 (2015).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Husemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Burfeind, K. G., Michaelis, K. A. & Marks, D. L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin. Cell Dev. Biol. 54, 42–52 (2016).
Braun, T. P. et al. Central nervous system inflammation induces muscle atrophy via activation of the hypothalamic-pituitary-adrenal axis. J. Exp. Med. 208, 2449–2463 (2011).
Anand, B. K. & Brobeck, J. R. Localization of a “feeding center” in the hypothalamus of the rat. Proc. Soc. Exp. Biol. Med. 77, 323–324 (1951).
Sohn, J. W. Network of hypothalamic neurons that control appetite. BMB Rep. 48, 229–233 (2015).
Ropelle, E. R. et al. A central role for neuronal adenosine 5’-monophosphate-activated protein kinase in cancer-induced anorexia. Endocrinology 148, 5220–5229 (2007).
Campos, C. A. et al. Cancer-induced anorexia and malaise are mediated by CGRP neurons in the parabrachial nucleus. Nat. Neurosci. 20, 934–942 (2017).
Cui, P. et al. Metabolic derangements of skeletal muscle from a murine model of glioma cachexia. Skelet. Muscle 9, 3 (2019).
Berghoff, A. S., Lassmann, H., Preusser, M. & Hoftberger, R. Characterization of the inflammatory response to solid cancer metastases in the human brain. Clin. Exp. Metastasis 30, 69–81 (2013).
Steeg, P. S. Targeting metastasis. Nat. Rev. Cancer 16, 201–218 (2016).
Fearon, K. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Prado, C. M., Birdsell, L. A. & Baracos, V. E. The emerging role of computerized tomography in assessing cancer cachexia. Curr. Opin. Support. Palliat. Care 3, 269–275 (2009).
Amthor, H. et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc. Natl Acad. Sci. USA 104, 1835–1840 (2007).
Temel, J. S. et al. Anamorelin in patients with non-small-cell lung cancer and cachexia (ROMANA 1 and ROMANA 2): results from two randomised, double-blind, phase 3 trials. Lancet Oncol. 17, 519–531 (2016).
Anker, M. S. et al. Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J. Cachexia Sarcopenia Muscle 10, 22–34 (2019).
Blum, D. et al. Validation of the consensus-definition for cancer cachexia and evaluation of a classification model–a study based on data from an international multicentre project (EPCRC-CSA). Ann. Oncol. 25, 1635–1642 (2014).
Vigano, A., Del Fabbro, E., Bruera, E. & Borod, M. The cachexia clinic: from staging to managing nutritional and functional problems in advanced cancer patients. Crit. Rev. Oncog. 17, 293–303 (2012).
Acknowledgements
The authors thank W. Ma, M. Kluger, G. Karsenty (CUIMC) and G. Miller (NYU) for helpful discussions. Funding was received from NCI CA231239, Pershing Square Sohn Prize, Irving Scholar Award, Interdisciplinary Research Initiatives Seed (IRIS) program, Developmental funds from NIH/NCI Cancer Center Support Grant P30CA013696 and The Irma T. Hirschl Monique Weill-Caulier Trust Award to S.A.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cancer thanks T. Guise, D. Lyden and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Biswas, A.K., Acharyya, S. Understanding cachexia in the context of metastatic progression. Nat Rev Cancer 20, 274–284 (2020). https://doi.org/10.1038/s41568-020-0251-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-020-0251-4
This article is cited by
-
Effect of combined Kinesiotaping and resistive exercise on muscle strength and quality of life in breast cancer survivors: a randomized clinical trial
Journal of the Egyptian National Cancer Institute (2024)
-
Leukemia inhibitory factor suppresses hepatic de novo lipogenesis and induces cachexia in mice
Nature Communications (2024)
-
LCN2 secreted by tissue-infiltrating neutrophils induces the ferroptosis and wasting of adipose and muscle tissues in lung cancer cachexia
Journal of Hematology & Oncology (2023)
-
An integrated PK/PD model investigating the impact of tumor size and systemic safety on animal survival in SW1990 pancreatic cancer xenograft
Acta Pharmacologica Sinica (2023)
-
Murine breast cancers disorganize the liver transcriptome in a zonated manner
Communications Biology (2023)