Abstract
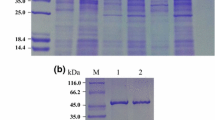
Site-directed mutagenesis was made to obtain a glucose isomerase (GI) working at high temperatures and low pH values and having high affinity to the substrate and more pH and termal stability in comparison with the native enzyme. For this purpose, GI gene from Geobacillus caldoxylosilyticus TK4 was cloned to pET-28a(+) vector and H99Q, V184T and D102N mutations were performed. Biochemical features of the recombinant and mutant enzymes were identified. These mutations enabled the mutant enzymes to increase the optimum temperature and KM and decrease the optimum pH and Vmax values of the reaction. Furthermore, the mutant proteins had more thermal and pH stability than that of the recombinant GI. The mutant enzymes showed the highest activities in the presence of Co2+, Cu2+ and Mn2+ and were more resistant to some metals than the recombinant GI. In conclusion, the site-directed mutations led to improve the performance of the recombinant enzyme, which means that the mutant enzyme (TK4GI mutation) may be practicable in high fructose corn syrup production process.






Similar content being viewed by others
REFERENCES
Fatima, B., Aftab, M.N., and Haq, I.U., J. Basic Microbiol., 2016, vol. 56, pp. 949–962.
Hartley, B.S., Hanlon, N., Jackson, R.J., and Rangarajan, M., BBA–Protein Struct. M., 2000, vol. 1543, pp. 294–335.
Bhosale, S.H., Rao, M.B., and Deshpande, V.V., Microbiol. Rev., 1996, vol. 60, pp. 280–300.
Brown, S.H., Sjøholm, C., and Kelly, R.M., Biotechnol. Bioeng., 1993, vol. 41, pp. 878–886.
Enzymes and Food Processing, Birch, G. G., Blackebrough, N., and Parker, J. K., Eds., London: Applied Science Publishers Ltd., 1981, pp. 51–72.
Kaneko, T., Takahashi, S., and Saito, K., Biosci. Biotechnol. Biochem., 2000, vol. 64, pp. 940–947.
Jeffries, T.W., Grigoriev, I.V., Grimwood, J., Laplaza, J.M., Aerts, A., Salamov, A., et al., Nat. Biotechnol., 2007, vol. 25, no. 3, pp. 319.
Lajoie, C.A., Kitner, J.B., Potochnik, S.J., Townsend, J.M., Beatty, C.C., and Kelly, C.J., Biotechnol. Progr., 2016, vol. 32, no. 5, pp. 1230–1237.
Joo, G.-J., Shin, J.-H., Heo, G.-Y., Kim, Y.-M., and Rhee, I.-K., J. Microbiol., 2005, vol. 43, no. 1, pp. 34–37.
Silva, C., Zangirolami, T., Rodrigues, J., Matugi, K., Giordano, R., and Giordano, R., Enzyme Microb. Technol., 2012, vol. 50, no. 1, pp. 35–42.
Li, W., Zhou, X., and Lu, P., Biotechnol. Adv., 2005, vol. 23, pp. 271–281.
Cha, J. and Batt, C.A., Mol. Cell., 1998, vol. 8, pp. 374–382.
Sriprapundh, D., Vieille, C., and Zeikus, J.G., Protein Eng., 2000, vol. 13, pp. 259–265.
Tsutakawa, S.E., Hura, G.L., Frankel, K.A., Cooper, P.K., and Tainer, J.A., J. Struct. Biol., 2007, vol. 158, pp. 214–223.
Dülger, S. Isolation, Characterization with Molecular Methods and Description of Thermophilic Bacteria from Çönen, Kestanbol and Diyadin Hot Springs,K.T.U., Trabzon, 2003.
Faiz, O., Colak, A., Kolcuoglu, Y., and Ertunga, N.S., Turk. J. Biochem., 2011, vol. 36, pp. 6–14.
Dische, Z. and Borenfreund, E., J. Biol. Chem., 1951, vol. 192, pp. 583–587.
Oz Tuncay, F., Colak, A., Yildirim Akatin, M., Kolcuoglu, Y., Saglam Ertunga, N., and Dokuzparmak, C., Rev. Roum. Chim., 2019, vol. 64, no. 1, pp. 73–82.
Vieille, C., Epting, K.L., Kelly, R.M., and Zeikus, J.G., Eur. J. Biochem., 2001, vol. 268, pp. 6291–6301.
Kovalevsky, A.Y., Hanson, L., Fisher, S.Z., Mustyakimov, M., Mason, S.A., Forsyth, V.T., et al., Structure, 2010, no. 6, pp. 688–699.
Lee, C., Bagdasarian, M., Meng, M., and Zeikus, J., J. Biol. Chem., 1990, vol. 265, pp. 19082–19090.
Xu, H., Shen, D., Wu, X.-Q., Liu, Z.-W., and Yang, Q.-H., J. Ind. Microbiol. Biotechnol., 2014, vol. 41, pp. 1581–1589.
Karaoglu, H., Yanmis, D., Sal, F.A., Celik, A., Canakci, S., and Belduz, A.O., J. Mol. Catal., B: Enzym., 2013, vol. 97, pp. 215–224.
Van Bastelaere, P., Kersters-Hilderson, H., and Lambeir, A., Biochem. J., 1995, vol. 307, pp. 135–142.
Liu, S.-Y., Wiegel, J., and Gherardini, F.C., J. Bacteriol., 1996, vol. 178, pp. 5938–5945.
Tewari, Y. and Goldberg, R.N., Appl. Biochem. Biotechnol., 1985, vol. 11, pp. 17–24.
Zhu, G.P., Xu, C., Teng, M.K., Tao, L.M., Zhu, X.Y., Wu, C.J., et al., Protein Eng., 1999, vol. 12, pp. 635–638.
Meng, M., Lee, C., Bagdasarian, M., and Zeikus, J.G., Proc. Natl. Acad. Sci. U. S. A., 1991, vol. 88, pp. 4015–4019.
Suekane, M., Tamura, M., and Tomimura, C., Agric. Biol. Chem., 1978, vol. 42, pp. 909–917.
Borgi, M.A., Srih-Belguith, K., Ali, M.B., Mezghani, M., Tranier, S., Haser, R., and Bejar, S., Biochimie, 2004, vol. 86, pp. 561–568.
Hlima, H.B., Aghajari, N., Ali, M.B., Haser, R., and Bejar, S., J. Ind. Microbiol. Biotechnol., 2012, vol. 39, pp. 537–546.
Jia, D.-X., Zhou, L., and Zheng, Y.-G., Enzyme Microb. Technol., 2017, vol. 99, pp. 1–8.
Kasumi, T., Mori, S., Kaneko, S., and Koyama, Y., J. Appl. Glycosci., 2011, vol. 59, pp. 43–46.
Lee, C. and Zeikus, J., Biochem. J., 1991, vol. 273, pp. 565–571.
Lee, C., Saha, B.C., and Zeikus, J.G., Appl. Environ. Microbiol., 1990, vol. 56, pp. 2895–2901.
Funding
This work was supported by the Scientific and Research Council of Turkey (TUBITAK) [no. 109T985].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Dokuzparmak, C., Colak, A., Kolcuoglu, Y. et al. Development of Some Properties of a Thermophilic Recombinant Glucose Isomerase by Mutation. Appl Biochem Microbiol 56, 164–172 (2020). https://doi.org/10.1134/S0003683820020052
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683820020052