Abstract
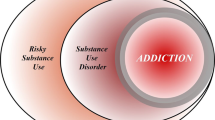
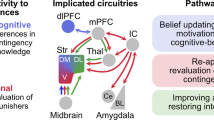
Compulsion is a cardinal symptom of drug addiction (severe substance use disorder). However, compulsion is observed in only a small proportion of individuals who repeatedly seek and use addictive substances. Here, we integrate accounts of the neuropharmacological mechanisms that underlie the transition to compulsion with overarching learning theories, to outline how compulsion develops in addiction. Importantly, we emphasize the conceptual distinctions between compulsive drug-seeking behaviour and compulsive drug-taking behaviour (that is, use). In the latter, an individual cannot stop using a drug despite major negative consequences, possibly reflecting an imbalance in frontostriatal circuits that encode reward and aversion. By contrast, an individual may compulsively seek drugs (that is, persist in seeking drugs despite the negative consequences of doing so) when the neural systems that underlie habitual behaviour dominate goal-directed behavioural systems, and when executive control over this maladaptive behaviour is diminished. This distinction between different aspects of addiction may help to identify its neural substrates and new treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5 (American Psychiatric Association, 2013).
Sanchis-Segura, C. & Spanagel, R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 11, 2–38 (2006).
Di Chiara, G. & Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl Acad. Sci. USA 85, 5274–5278 (1988).
Lammel, S. et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature 491, 212–217 (2012).
Yang, H. et al. Nucleus accumbens subnuclei regulate motivated behavior via direct inhibition and disinhibition of VTA dopamine subpopulations. Neuron 97, 434–449 e434 (2018).
Kelley, A. & Berridge, K. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 22, 3306–3311 (2002).
Stuber, G. D. & Wise, R. A. Lateral hypothalamic circuits for feeding and reward. Nat. Neurosci. 19, 198–205 (2016).
Balleine, B. Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Phys. Behav. 86, 717–730 (2005).
Naqvi, N., Rudrauf, D., Damasio, H. & Bechara, A. Damage to the insula disrupts addiction to cigarette smoking. Science 315, 531–534 (2007).
Lüscher, C. & Ungless, M. The mechanistic classification of addictive drugs. PLoS Med. 3, e437 (2006).
Abrahao, K. P., Salinas, A. G. & Lovinger, D. M. Alcohol and the brain: neuronal molecular targets, synapses, and circuits. Neuron 96, 1223–1238 (2017).
Johnson, S. & North, R. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 12, 483–488 (1992).
Patriarchi, T. et al. Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360, eaat4422 (2018).
Corre, J. et al. Dopamine neurons projecting to medial shell of the nucleus accumbens drive heroin reinforcement. eLife https://doi.org/10.7554/eLife.39945 (2018).
Ettenberg, A., Pettit, H. O., Bloom, F. E. & Koob, G. F. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology 78, 204–209 (1982).
Pettit, H., Ettenberg, A., Bloom, F. & Koob, G. Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin self-administration in rats. Psychopharmacology 84, 167–173 (1984).
Ting, A. K. R. & van der Kooy, D. The neurobiology of opiate motivation. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a012096 (2012).
Daglish, M. R. et al. Brain dopamine response in human opioid addiction. Br. J. Psychiatry 193, 65–72 (2008).
Nutt, D. J., Lingford-Hughes, A., Erritzoe, D. & Stokes, P. R. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci.16, 305–312 (2015).
Saal, D., Dong, Y., Bonci, A. & Malenka, R. C. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37, 577–582 (2003).
Ungless, M. A., Whistler, J. L., Malenka, R. C. & Bonci, A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–587 (2001). Original demonstration of drug-evoked synaptic plasticity in VTA dopamine neurons after a single injection of cocaine.
Brown, M. T. et al. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS One 5, e15870 (2010).
Mameli, M. et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci. 12, 1036–1041 (2009).
Pascoli, V. et al. Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509, 459–464 (2014).
Pascoli, V., Terrier, J., Hiver, A. & Lüscher, C. Sufficiency of mesolimbic dopamine neuron stimulation for the progression to addiction. Neuron 88, 1054–1066 (2015).
Terrier, J., Lüscher, C. & Pascoli, V. Cell-type specific insertion of GluA2-lacking AMPARs with cocaine exposure leading to sensitization, cue-induced seeking, and incubation of craving. Neuropsychopharmacology 41, 1779–1789 (2016).
Koob, G. & Le Moal, M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24, 97–129 (2001).
Koob, G. F. & Volkow, N. D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773 (2016).
Koob, G. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 56 (Suppl. 1), 18–31 (2009).
Zhu, Y., Wienecke, C. F., Nachtrab, G. & Chen, X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature 530, 219–222 (2016).
Boulos, L. J. et al. Mu opioid receptors in the medial habenula contribute to naloxone aversion. Neuropsychopharmacology 45, 247–255 (2020).
Boileau, I. et al. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch. Gen. Psychiatry 63, 1386–1395 (2006).
Wong, D. et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology 31, 2716–2727 (2006).
Trifilieff, P., Ducrocq, F., van der Veldt, S. & Martinez, D. Blunted dopamine transmission in addiction: potential mechanisms and implications for behavior. Semin. Nucl. Med. 47, 64–74 (2017).
Volkow, N. D. et al. Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Mol. Psychiatry 19, 1037–1043 (2014).
Balleine, B. W. & O’Doherty, J. P. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69 (2010).
Ersche, K. D. et al. Carrots and sticks fail to change behavior in cocaine addiction. Science 352, 1468–1471 (2016).
Voon, V. et al. Disorders of compulsivity: a common bias towards learning habits. Mol. Psychiatry 20, 345–352 (2014).
Luijten, M. et al. Goal-directed and habitual control in smokers. Nicotine Tob. Res. 22, 188–195 (2019).
Sjoerds, Z. et al. Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl. Psychiatry 3, e337 (2013).
Ersche, K. D., Williams, G. B., Robbins, T. W. & Bullmore, E. T. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Curr. Opin. Neurobiol. 23, 615–624 (2013).
Everitt, B. J. & Robbins, T. W. Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 67, 23–50 (2016).
Goldstein, R. & Volkow, N. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 159, 1642–1652 (2002).
Ersche, K. et al. Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 134, 2013–2024 (2011). Demonstration that neural mechanisms of impaired top-down inhibitory control in human stimulant addiction are pre-existing rather than caused by drug exposure.
Ersche, K. et al. Abnormal brain structure implicated in stimulant drug addiction. Science 335, 601–604 (2012).
Anthony, J. C., Warner, L. A. & Kessler, R. C. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 2, 244–268 (1994).
Deroche-Gamonet, V., Belin, D. & Piazza, P. V. Evidence for addiction-like behavior in the rat. Science 305, 1014–1017 (2004). First demonstration of individual differences using an addiction model in rats.
Pascoli, V. et al. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature 564, 366–371 (2018). Demonstration of apparent ‘gain of function’ in orbitofrontal–striatal circuit mediating compulsive reward-related behaviour caused by selective optogenetic stimulation of VTA dopamine neurons.
Kasanetz, F. et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 328, 1709–1712 (2010).
Hu, Y. Z. et al. Compulsive drug use is associated with imbalance of orbitofrontal- and prelimbic-striatal circuits in punishment-resistant individuals. Proc. Natl Acad. Sci. USA 116, 9066–9071 (2019).
Augier, E. et al. A molecular mechanism for choosing alcohol over an alternative reward. Science 360, 1321–1326 (2018). Individual differences in choice preference for adulterated alcohol over saccharin predict subsequent compulsive drinking in rats and correlate with downregulation of GABAergic function in the central amygdala, relevant to parallel human studies of alcohol dependence.
Siciliano, C. A. et al. A cortical-brainstem circuit predicts and governs compulsive alcohol drinking. Science 366, 1008–1012 (2019).
Lee, H., Gallagher, M. & Holland, P. The central amygdala projection to the substantia nigra reflects prediction error information in appetitive conditioning. Learn. Mem. 17, 531–538 (2010).
Wall, N. R. et al. Complementary genetic targeting and monosynaptic input mapping reveal recruitment and refinement of distributed corticostriatal ensembles by cocaine. Neuron 104, 916–930 e915 (2019).
Hirokawa, J., Vaughan, A., Masset, P., Ott, T. & Kepecs, A. Frontal cortex neuron types categorically encode single decision variables. Nature 576, 446–451 (2019).
Whitelaw, R. B., Markou, A., Robbins, T. W. & Everitt, B. J. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology 127, 213–224 (1996).
Ito, R., Robbins, T. & Everitt, B. Differential control over cocaine-seeking behavior by nucleus accumbens core and shell. Nat. Neurosci. 7, 389–397 (2004).
Di Ciano, P. & Everitt, B. J. Direct interactions between the basolateral amygdala and nucleus accumbens core underlie cocaine-seeking behavior by rats. J. Neurosci. 24, 7167–7173 (2004).
Cador, M., Robbins, T. W. & Everitt, B. J. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience 30, 77–86 (1989).
Mcfarland, K. & Kalivas, P. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 21, 8655–8663 (2001).
Pickens, C. et al. Neurobiology of the incubation of drug craving. Trends Neurosci. 34, 411–420 (2011).
Pascoli, V., Turiault, M. & Lüscher, C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature 481, 71–75 (2011). First demonstration that the reversal of drug-evoked synaptic potentiation abolishes adaptive behaviour.
Shen, C. J. et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 25, 337–349 (2019).
Kim, J., Pignatelli, M., Xu, S., Itohara, S. & Tonegawa, S. Antagonistic negative and positive neurons of the basolateral amygdala. Nat. Neurosci. 19, 1636–1646 (2016).
Everitt, B. J. & Robbins, T. W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 8, 1481–1489 (2005).
Balleine, B., Liljeholm, M. & Ostlund, S. The integrative function of the basal ganglia in instrumental conditioning. Behav. Brain Res. 199, 43–52 (2009).
Hilario, M. R. & Costa, R. M. High on habits. Front. Neurosci. 2, 208–217 (2008).
Gremel, C. M. et al. Endocannabinoid modulation of orbitostriatal circuits gates habit formation. Neuron 90, 1312–1324 (2016). Molecular basis of possible top-down prefrontal circuitry regulating habit formation.
Murray, J. E., Belin, D. & Everitt, B. J. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology 37, 2456–2466 (2012).
Corbit, L., Nie, H. & Janak, P. Habitual alcohol seeking: time course and the contribution of subregions of the dorsal striatum. Biol. Psychiatry 72, 389–395 (2012). Experimental evidence for the involvement of the dorsal striatum in alcohol-seeking behaviour.
Hellard, E. R. et al. Optogenetic control of alcohol-seeking behavior via the dorsomedial striatal circuit. Neuropharmacology 155, 89–97 (2019).
Ostlund, S. & Balleine, B. On habits and addiction: an associative analysis of compulsive drug seeking. Drug. Discov. Today Dis. Model. 5, 235–245 (2008).
Vollstadt-Klein, S. et al. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction 105, 1741–1749 (2010).
Zhou, A. et al. Cue reactivity in the ventral striatum characterizes heavy cannabis use, whereas reactivity in the dorsal striatum mediates dependent use. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 751–762 (2019).
Volkow, N. D. et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 26, 6583–6588 (2006).
Cuzon Carlson, V. C. et al. Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36, 2513–2528 (2011).
Ito, R., Dalley, J., Robbins, T. & Everitt, B. Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. J. Neurosci. 22, 6247–6253 (2002).
Vanderschuren, L., Di Ciano, P. & Everitt, B. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J. Neurosci. 25, 8665–8670 (2005).
Hodebourg, R. et al. Heroin seeking becomes dependent on dorsal striatal dopaminergic mechanisms and can be decreased by N-acetylcysteine. Eur. J. Neurosci. 50, 2036–2044 (2019).
Zapata, A., Minney, V. & Shippenberg, T. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J. Neurosci. 30, 15457–15463 (2010).
Smith, R. J. & Laiks, L. S. Behavioral and neural mechanisms underlying habitual and compulsive drug seeking. Prog. Neuropsychopharmacol. Biol. Psychiatry 87, 11–21 (2018).
Giuliano, C., Belin, D. & Everitt, B. J. Compulsive alcohol seeking results from a failure to disengage dorsolateral striatal control over behavior. J. Neurosci. 39, 1744–1754 (2019).
Everitt, B. J. & Robbins, T. W. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. https://doi.org/10.1016/j.neubiorev.2013.02.010 (2013).
Haber, S., Fudge, J. & McFarland, N. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 20, 2369–2382 (2000). Groundbreaking neuroanatomical study providing an anatomical substrate for the transition to compulsive drug-seeking behaviour, based on discovery of a spiralling organization of striatal feed-forward circuitry.
Ikemoto, S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 56, 27–78 (2007).
Belin, D. & Everitt, B. J. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron 57, 432–441 (2008). A critical test of the relative roles of the ventral striatum and the dorsal striatum in the transition to compulsive drug-seeking behaviour.
Willuhn, I., Burgeno, L. M., Everitt, B. J. & Phillips, P. E. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc. Natl Acad. Sci. USA 109, 20703–20708 (2012).
Wouterlood, F. G. et al. Mesencephalic dopamine neurons interfacing the shell of nucleus accumbens and the dorsolateral striatum in the rat. J. Neurosci. Res. 96, 1518–1542 (2018).
Bocklisch, C. et al. Cocaine disinhibits dopamine neurons by potentiation of GABA transmission in the ventral tegmental area. Science 341, 1521–1525 (2013).
Cardinal, R. The contribution of the amygdala, nucleus accumbens, and prefrontal cortex to emotion and motivated behaviour. Int. Congr. Ser. 1250, 347–370 (2003).
Corbit, L. & Balleine, B. The general and outcome-specific forms of Pavlovian-instrumental transfer are differentially mediated by the nucleus accumbens core and shell. J. Neurosci. 31, 11786–11794 (2011).
Murray, J. E. et al. Basolateral and central amygdala differentially recruit and maintain dorsolateral striatum-dependent cocaine-seeking habits. Nat. Commun.6, 10088 (2015).
Lingawi, N. W. & Balleine, B. W. Amygdala central nucleus interacts with dorsolateral striatum to regulate the acquisition of habits. J. Neurosci. 32, 1073–1081 (2012).
El-Amamy, H. & Holland, P. Dissociable effects of disconnecting amygdala central nucleus from the ventral tegmental area or substantia nigra on learned orienting and incentive motivation. Eur. J. Neurosci. 25, 1557–1567 (2007).
Urcelay, G. P. & Jonkman, S. Delayed rewards facilitate habit formation. J. Exp. Psychol. Anim. Learn. Cogn. 45, 413–421 (2019).
Nelson, A. & Killcross, S. Amphetamine exposure enhances habit formation. J. Neurosci. 26, 3805–3812 (2006).
Schmitzer-Torbert, N. et al. Post-training cocaine administration facilitates habit learning and requires the infralimbic cortex and dorsolateral striatum. Neurobiol. Learn. Mem. 118, 105–112 (2015).
Hogarth, L., Balleine, B., Corbit, L. & Killcross, S. Associative learning mechanisms underpinning the transition from recreational drug use to addiction. Ann. N. Y. Acad. Sci. 1282, 12–24 (2013).
Dickinson, A., Wood, N. & Smith, J. Alcohol seeking by rats: action or habit? Q. J. Exp. Psychol. B 55, 331–348 (2002).
Miles, F., Everitt, B. & Dickinson, A. Oral cocaine seeking by rats: action or habit? Behav. Neurosci. 117, 927–938 (2003).
Clemens, K. J., Castino, M. R., Cornish, J. L., Goodchild, A. K. & Holmes, N. M. Behavioral and neural substrates of habit formation in rats intravenously self-administering nicotine. Neuropsychopharmacology 39, 2584–2593 (2014).
Malvaez, M. & Wassum, K. M. Regulation of habit formation in the dorsal striatum. Opin. Behav. Sci. 20, 67–74 (2018).
Schoenbaum, G. & Shaham, Y. The role of orbitofrontal cortex in drug addiction: a review of preclinical studies. Biol. Psychiatry 63, 256–262 (2008).
Hampson, R., Porrino, L., Opris, I., Stanford, T. & Deadwyler, S. Effects of cocaine rewards on neural representations of cognitive demand in nonhuman primates. Psychopharmacology 213,105–118 (2010).
Crombag, H., Gorny, G., Li, Y., Kolb, B. & Robinson, T. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb. Cortex 15, 341–348 (2005).
Morein-Zamir, S. & Robbins, T. W. Fronto-striatal circuits in response-inhibition: relevance to addiction. Brain Res. 21, 488–497 (2014).
Rogers, R. D. et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 20, 322–339 (1999).
Bechara, A. et al. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia 39, 376–389 (2001).
Hardwick, R. M., Forrence, A. D., Krakauer, J. W. & Haith, A. M. Time-dependent competition between goal-directed and habitual response preparation. Nat. Hum. Behav. 3, 1252–1262 (2019).
Jonkman, S., Pelloux, Y. & Everitt, B. J. Drug intake is sufficient, but conditioning is not necessary for the emergence of compulsive cocaine seeking after extended self-administration. Neuropsychopharmacology 37, 1612–1619 (2012).
Belin, D., Mar, A. C., Dalley, J. W., Robbins, T. W. & Everitt, B. J. High impulsivity predicts the switch to compulsive cocaine-taking. Science 320, 1352–1355 (2008).
Belin, D., Belin-Rauscent, A., Everitt, B. J. & Dalley, J. W. In search of predictive endophenotypes in addiction: insights from preclinical research. Genes Brain Behav. 15, 74–88 (2016).
Marchant, N. J., Campbell, E. J. & Kaganovsky, K. Punishment of alcohol-reinforced responding in alcohol preferring P rats reveals a bimodal population: Implications for models of compulsive drug seeking. Prog. Neuropsychopharmacol. Biol. Psychiatry 87, 68–77 (2018).
Pelloux, Y., Murray, J. E. & Everitt, B. J. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur. J. Neurosci. 38, 3018–3026 (2013).
Zapata, A., Hwang, E. K. & Lupica, C. R. lateral habenula involvement in impulsive cocaine seeking. Neuropsychopharmacology 42, 1103–1112 (2017).
Matsumoto, M. & Hikosaka, O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447, 1111–1115 (2007).
Chen, B. et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–362 (2013).
Pelloux, Y., Everitt, B. J. & Dickinson, A. Compulsive drug seeking by rats under punishment: effects of drug taking history. Psychopharmacology 194, 127–137 (2007).
Fan, X. C. et al. Hypersensitivity of prelimbic cortex neurons contributes to aggravated nociceptive responses in rats with experience of chronic inflammatory pain. Front. Mol. Neurosci. 11, 85 (2018).
Jonkman, S., Pelloux, Y. & Everitt, B. J. Differential roles of the dorsolateral and midlateral striatum in punished cocaine seeking. J. Neurosci. 32, 4645–4650 (2012).
Renteria, R., Baltz, E. T. & Gremel, C. M. Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat. Commun. 9, 211 (2018).
Harada, M., Hiver, A., Pascoli, V. & Lüscher, C. Cortico-striatal synaptic plasticity underlying compulsive reward seeking. bioRxiv https://doi.org/10.1101/789495 (2019).
Rogers, R. D. & Robbins, T. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr. Opin. Neurobiol. 11, 250–257 (2001).
Belin, D., Belin-Rauscent, A., Murray, J. E. & Everitt, B. J. Addiction: failure of control over maladaptive habits. Curr. Opin. Biol. 23, 564–572 (2013).
Ahmed, S. H. Trying to make sense of rodents’ drug choice behavior. Neuropsychopharmacol. Biol. Psychiatry 87, 3–10 (2018).
Cantin, L. et al. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One 5, e11592 (2010).
Higgins, S. T., Heil, S. H. & Lussier, J. P. Clinical implications of reinforcement as a determinant of substance use disorders. Annu. Rev. Psychol. 55, 431–461 (2004).
Davis, D. R. et al. A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Prev. Med. 92, 36–46 (2016).
Hogarth, L. et al. Intact goal-directed control in treatment-seeking drug users indexed by outcome-devaluation and Pavlovian to instrumental transfer: critique of habit theory. Eur. J. Neurosci. 50, 2513–2525 (2019).
Momennejad, I. et al. The successor representation in human reinforcement learning. Nat. Hum. Behav. 1, 680–692 (2017).
Singer, B. F., Fadanelli, M., Kawa, A. B. & Robinson, T. E. Are cocaine-seeking “habits” necessary for the development of addiction-like behavior in rats? J. Neurosci. 38, 60–73 (2018).
Colwill, R. M. & Rescorla, R. A. Associative structures in instrumental learning. Psychol. Learn. Motiv. 20, 55–104 (1986).
Holland, P. Relations between Pavlovian-instrumental transfer and reinforcer devaluation. J. Exp. Psychol. Anim. Behav. Process. 30, 104–117 (2004).
Kosaki, Y. & Dickinson, A. Choice and contingency in the development of behavioral autonomy during instrumental conditioning. J. Exp. Psychol. Anim. Behav. Process. 36, 334–342 (2010).
Weiss, F., Markou, A., Lorang, M. T. & Koob, G. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 593, 314–318 (1992).
Mateo, Y., Lack, C., Morgan, D., Roberts, D. & Jones, S. Reduced dopamine terminal function and insensitivity to cocaine following cocaine binge self-administration and deprivation. Neuropsychopharmacology 30, 1455–1463 (2005).
Volkow, N. D. & Fowler, J. S. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cortex 10, 318–325 (2000).
Pelloux, Y. & Baunez, C. Deep brain stimulation for addiction: why the subthalamic nucleus should be favored. Curr. Opin. Neurobiol. 23, 713–720 (2013).
Terraneo, A. et al. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: a pilot study. Eur. Neuropsychopharmacol. 26, 37–44 (2016).
Weeks, J. R. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science 138, 143–144 (1962).
Dickinson, A. Conditioning and associative learning. Br. Med. Bull. 37, 165–168 (1981).
Wise, R. A. Role of brain dopamine in food reward and reinforcement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1149–1158 (2006).
Koob, G. F. New dimensions in human laboratory models of addiction. Addict. Biol. 14, 1–8 (2009).
Ahmed, S. H. & Koob, G. Transition from moderate to excessive drug intake: change in hedonic set point. Science 282, 298–300 (1998).
Wolffgramm, J. An ethopharmacological approach to the development of drug addiction. Neurosci. Biobehav. Rev. 15, 515–519 (1991).
Ahmed, S. H. & Koob, G. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology 146, 303–312 (1999).
O’Dell, L. E., Roberts, A. J., Smith, R. T. & Koob, G. F. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol. Clin. Exp. Res. 28, 1676–1682 (2004).
Paterson, N. E. & Markou, A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport 14, 2229–2232 (2003).
Koob, G. Negative reinforcement in drug addiction: the darkness within. Curr. Opin. Neurobiol. 23, 559–563 (2013).
Kawa, A. B., Bentzley, B. S. & Robinson, T. E. Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233, 3587–3602 (2016).
Lesscher, H. & Vanderschuren, L. Compulsive drug use and its neural substrates. Rev. Neurosci. 23, 731–745 (2012).
Vengeliene, V., Celerier, E., Chaskiel, L., Penzo, F. & Spanagel, R. Compulsive alcohol drinking in rodents. Addict. Biol. 14, 384–396 (2009).
Tornatzky, W. & Miczek, K. A. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology 148, 289–298 (2000).
Belin, D., Balado, E., Piazza, P. & Deroche-Gamonet, V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol. Psychiatry 65, 863–868 (2009).
Seif, T. et al. Cortical activation of accumbens hyperpolarization-active NMDARs mediates aversion-resistant alcohol intake. Nat. Neurosci. 16, 1094–1100 (2013).
Torres, O. V. et al. Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behav. Brain Res. 326, 265–271 (2017).
Belin, D., Deroche-Gamonet, V. & Jaber, M. Cocaine-induced sensitization is associated with altered dynamics of transcriptional responses of the dopamine transporter, tyrosine hydroxylase, and dopamine D2 receptors in C57Bl/6J mice. Psychopharmacology 193, 567–578 (2007).
Olmstead, M., Lafond, M., Everitt, B. J. & Dickinson, A. Cocaine seeking by rats is a goal-directed action. Behav. Neurosci. 115, 394–402 (2001).
Olmstead, M., Parkinson, J., Miles, F., Everitt, B. J. & Dickinson, A. Cocaine-seeking by rats: regulation, reinforcement and activation. Psychopharmacology 152, 123–131 (2000).
Goldberg, S. R. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J. Pharmacol. Exp. Ther. 186, 18–30 (1973).
Everitt, B. J. & Robbins, T. W. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology 153, 17–30 (2000).
Giuliano, C. et al. Evidence for a long-lasting compulsive alcohol seeking phenotype in rats. Neuropsychopharmacology 43, 728–738 (2018).
Everitt, B. J. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories-indications for novel treatments of addiction. Eur. J. Neurosci. 40, 2163–2182 (2014).
Grimm, J. W., Hope, B. T., Wise, R. A. & Shaham, Y. Incubation of cocaine craving after withdrawal. Nature 412, 141–142 (2001).
Venniro, M., Caprioli, D. & Shaham, Y. Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog. Brain Res. 224, 25–52 (2016).
Wolf, M. E. Synaptic mechanisms underlying persistent cocaine craving. Nat. Rev. Neurosci. 17, 351–365 (2016).
Kalivas, P. W. & McFarland, K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168, 44–56 (2003).
Szumlinski, K. et al. Homer isoforms differentially regulate cocaine-induced neuroplasticity. Neuropsychopharmacology 31, 768–777 (2006).
Kalivas, P. W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 10, 561–572 (2009).
Vanderschuren, L. J. & Everitt, B. J. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305, 1017–1019 (2004).
Ostlund, S. & Balleine, B. Differential involvement of the basolateral amygdala and mediodorsal thalamus in instrumental action selection. J. Neurosci. 28, 4398–4405 (2008).
Cardinal, R., Parkinson, J. A., Hall, J. & Everitt, B. J. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 26, 321–352 (2002).
Kuhn, B. N., Campus, P. & Flagel, S. B. The Neurobiological Mechanisms Underlying Sign-Tracking Behavior (Michigan Publishing, 2018).
Yin, H. H., Ostlund, S. B., Knowlton, B. J. & Balleine, B. W. The role of the dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 22, 513–523 (2005).
Hart, G., Bradfield, L. A., Fok, S. Y., Chieng, B. & Balleine, B. W. The bilateral prefronto-striatal pathway is necessary for learning new goal-directed actions. Curr. Biol. 28, 2218–2229.e7 (2018).
Balleine, B., Leung, B. & Ostlund, S. The orbitofrontal cortex, predicted value, and choice. Ann. N. Y. Acad. Sci. 1239, 43–50 (2011).
Parkes, S. L. et al. Insular and ventrolateral orbitofrontal cortices differentially contribute to goal-directed behavior in rodents. Cereb. Cortex 28, 2313–2325 (2018).
Bradfield, L. A., Dezfouli, A., van Holstein, M., Chieng, B. & Balleine, B. W. Medial orbitofrontal cortex mediates outcome retrieval in partially observable task situations. Neuron 88, 1268–1280 (2015).
Murray, E. A. & Rudebeck, P. H. Specializations for reward-guided decision-making in the primate ventral prefrontal cortex. Nat. Rev. Neurosci. 19, 404–417 (2018).
Dickinson, A. Actions and habits: the development of behavioural autonomy. Philos. Trans. R. Soc. Lond. B 308, 67–78 (1985).
Wood, W. & Runger, D. Psychology of habit. Annu. Rev. Psychol. 67, 289–314 (2016).
Yin, H. H., Knowlton, B. J. & Balleine, B. W. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur. J. Neurosci. 19, 181–189 (2004).
Killcross, S. & Coutureau, E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb. Cortex 13, 400–408 (2003).
Smith, K. S., Virkud, A., Deisseroth, K. & Graybiel, A. M. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc. Natl Acad. Sci. USA 109, 18932–18937 (2012).
Smith, K. S. & Graybiel, A. M. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron 79, 361–374 (2013).
Cardinal, R. N. & Cheung, T. H. Nucleus accumbens core lesions retard instrumental learning and performance with delayed reinforcement in the rat. BMC Neurosci. 6, 9 (2005).
Parkinson, J. A., Olmstead, M. C., Burns, L. H., Robbins, T. W. & Everitt, B. J. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine. J. Neurosci. 19, 2401–2411 (1999).
Heymann, G. et al. Synergy of distinct dopamine projection populations in behavioral reinforcement. Neuron 105, 909–920.e5 (2019).
Acknowledgements
The authors acknowledge the Swiss National Science Foundation and the European Research Council (C.L.) as well as the UK Medical Research Council (grant MR/N02530X/1) and the Wellcome Trust (Investigator Award WT 104631/Z/14/Z/) for financial support (B.J.E., T.W.R.). The authors thank D. Belin and M. Loureiro for help with the figures.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the article’s content, wrote the article, and reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
C.L. has no competing interests. T.W.R. consults for Cambridge Cognition, Unilever, Cassava and Greenfield BioVentures. He holds research grants from Shionogi & Co., Ltd and GlaxoSmithKline plc., and receives royalties from Cambridge Cognition for CANTAB. He also receives editorial honoraria from Springer and Elsevier. B.J.E. has no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Titrated
-
Adjusted in intensity, to measure the effect of a stimulus on behaviour.
- Escalation of drug intake
-
Increase of drug intake during extended (long-access) periods of self-administration.
- Reward thresholds
-
The minimal stimulation intensities required to produce a reinforcing effect.
- Natural reinforcers
-
Rewards such as food and sex that motivate behaviour in animals and humans. They may be distinguished from artificial rewards such as addictive drugs that may nevertheless depend on the same neural systems in the brain.
- Interoceptive
-
Relating to internal, or bodily, states that are interpreted by the brain through a process called ‘interoception’. In the case of drugs, bodily changes (such as increases in heart rate caused by stimulants) are an important component of the subjective effects of drugs.
- Stimulant
-
A drug that increases arousal and activity. Amphetamine is a typical example of a stimulant, sometimes called a ‘psychomotor stimulant’.
- Locomotor sensitization
-
Enhanced motor responses to the same does of a stimulant drug that follows intermittent, repeated dosing.
- Cue-elicited reinstatement
-
Reinstatement of performance of previously extinguished drug-taking responses, supported by drug cues acting as conditioned reinforcers.
- Extended amygdala
-
Neuroanatomical term that includes the centromedial amygdala, bed nucleus of the stria terminalis and, according to some, the shell of the nucleus accumbens and a group of neurons in the basal forebrain that links these structures.
- Dysphoria
-
A state of unhappiness or suboptimal mood in humans.
- Pavlovian–instrumental transfer
-
(PIT). Transfer of learning whereby conditioned stimuli associated with a reward can increase a separately trained instrumental response for that reward (specific transfer) or for other rewards (general transfer).
- Sign tracking
-
Behaviour whereby the animal approaches a conditioned stimulus predictive of reward — as opposed to approaching a reward (or goal) directly (‘goal tracking’).
- Contingency degradation
-
Degradation of the predictive relationship between responses and outcomes; for example, by presentation of ‘free’ (that is, response-independent) outcomes or extinction.
- Quinine
-
A bitter crystalline compound present in cinchona bark that is used to adulterate an otherwise readily ingested liquid.
- Progressive ratio schedule
-
A behavioural procedure whereby the number of required responses increases after each reward delivery. The number of responses at which the animal ceases to respond is called the ‘break point’.
- Analgesic
-
Relating to the effects of a drug that relieves pain (for example, morphine).
- Value updating
-
The perception of a change in value of a reinforcer after an antecedent manipulation; for example, the value of food is decreased following ingestion of the food to satiety.
- Incubation of cocaine craving
-
The increase in instrumental responding for a drug-associated conditioned stimulus that occurs the longer the period of abstinence from drug taking.
- Direct pathway
-
Projection of dopamine D1 receptor-expressing medium spiny neurons in the striatum to the midbrain. The indirect pathway involves a striatal projection of dopamine D2 receptor-expressing medium spiny neurons to the pallidum.
- Contingency management
-
A behavioural modification intervention that reinforces desired behaviour (such as abstinence) with incentives.
- Reversal learning
-
Learning of a reversal of the reward contingencies of two options, reflecting behavioural adaptation to environmental change.
Rights and permissions
About this article
Cite this article
Lüscher, C., Robbins, T.W. & Everitt, B.J. The transition to compulsion in addiction. Nat Rev Neurosci 21, 247–263 (2020). https://doi.org/10.1038/s41583-020-0289-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41583-020-0289-z
This article is cited by
-
Different mechanisms underlie compulsive alcohol self-administration in male and female rats
Biology of Sex Differences (2024)
-
Incubation of methamphetamine craving in punishment-resistant individuals is associated with activation of specific gene networks in the rat dorsal striatum
Molecular Psychiatry (2024)
-
Delay of punishment highlights differential vulnerability to developing addiction-like behavior toward sweet food
Translational Psychiatry (2024)
-
Transcriptomic effects of paternal cocaine-seeking on the reward circuitry of male offspring
Translational Psychiatry (2024)
-
High frequency deep brain stimulation of the dorsal raphe nucleus prevents methamphetamine priming-induced reinstatement of drug seeking in rats
Translational Psychiatry (2024)