Abstract
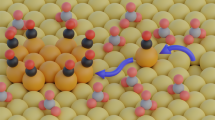
The efficient electrochemical conversion of CO2 provides a route to fuels and feedstocks. Copper catalysts are well-known to be selective to multicarbon products, although the role played by the surface architecture and the presence of oxides is not fully understood. Here we report improved efficiency towards ethanol by tuning the morphology and oxidation state of the copper catalysts through pulsed CO2 electrolysis. We establish a correlation between the enhanced production of C2+ products (76% ethylene, ethanol and n-propanol at −1.0 V versus the reversible hydrogen electrode) and the presence of (100) terraces, Cu2O and defects on Cu(100). We monitored the evolution of the catalyst morphology by analysis of cyclic voltammetry curves and ex situ atomic force microscopy data, whereas the chemical state of the surface was examined via quasi in situ X-ray photoelectron spectroscopy. We show that the continuous regeneration of defects and Cu(i) species synergistically favours C–C coupling pathways.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available in the paper, Supplementary Information and Source Data. Additional datasets related to this study are available from the corresponding author on reasonable request.
References
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Zhang, W. et al. Progress and perspective of electrocatalytic CO2 reduction for renewable carbonaceous fuels and chemicals. Adv. Sci. 5, 1700275 (2018).
Hori, Y. Electrochemical CO2 reduction on metal electrodes. Mod. Asp. Electrochem. 42, 89–189 (2008).
Gattrell, M., Gupta, N. & Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 594, 1–19 (2006).
Gao, D., Arán-Ais, R. M., Jeon, H. S. & Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2, 198–210 (2019).
Singh, M. R., Clark, E. L. & Bell, A. T. Thermodynamic and achievable efficiencies for solar-driven electrochemical reduction of carbon dioxide to transportation fuels. Proc. Natl Acad. Sci. USA 112, E6111–E6118 (2015).
Schouten, K. J. P., Kwon, Y., van der Ham, C. J. M., Qin, Z. & Koper, M. T. M. A new mechanism for the selectivity to C1 and C2 species in the electrochemical reduction of carbon dioxide on copper electrodes. Chem. Sci. 2, 1902–1909 (2011).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of CO2 into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Arán-Ais, R. M., Gao, D. & Roldan Cuenya, B. Structure- and electrolyte-sensitivity in CO2 electroreduction. Acc. Chem. Res. 51, 2906–2917 (2018).
Durand, W. J., Peterson, A. A., Studt, F., Abild-Pedersen, F. & Nørskov, J. K. Structure effects on the energetics of the electrochemical reduction of CO2 by copper surfaces. Surf. Sci. 605, 1354–1359 (2011).
Montoya, J. H., Shi, C., Chan, K. & Nørskov, J. K. Theoretical insights into a CO dimerization mechanism in CO 2 electroreduction. J. Phys. Chem. Lett. 6, 2032–2037 (2015).
Calle-Vallejo, F. & Koper, M. T. M. Theoretical considerations on the electroreduction of CO to C2 Species on Cu(100) electrodes. Angew. Chem. Int. Ed. 52, 7282–7285 (2013).
Bagger, A., Ju, W., Varela, A. S., Strasser, P. & Rossmeisl, J. Electrochemical CO2 reduction: classifying Cu facets. ACS Catal. 9, 7894–7899 (2019).
Hori, Y., Takahashi, I., Koga, O. & Hoshi, N. Selective formation of C2 compounds from electrochemical reduction of CO2 at a series of copper single crystal electrodes. J. Phys. Chem. B 106, 15–17 (2002).
Hori, Y., Takahashi, I., Koga, O. & Hoshi, N. Electrochemical reduction of carbon dioxide at various series of copper single crystal electrodes. J. Mol. Catal. A Chem. 199, 39–47 (2003).
Huang, Y., Handoko, A. D., Hirunsit, P. & Yeo, B. S. Electrochemical reduction of CO2 using copper single-crystal surfaces: effects of CO* coverage on the selective formation of ethylene. ACS Catal. 7, 1749–1756 (2017).
Zhou, Y. et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 10, 974–980 (2018).
Eilert, A. et al. Subsurface oxygen in oxide-derived copper electrocatalysts for carbon dioxide reduction. J. Phys. Chem. Lett. 8, 285–290 (2017).
Xiao, H., Goddard, W., Cheng, T. & Liu, Y. Cu metal embedded in oxidized matrix catalyst to promote CO2 activation and CO dimerization for electrochemical reduction of CO2. Proc. Natl Acad. Sci. USA 114, 6685–6688 (2017).
Mistry, H. et al. Highly selective plasma-activated copper catalysts for CO2 reduction to ethylene. Nat. Commun. 7, 12123 (2016).
Gao, D. et al. Plasma-activated copper nanocube catalysts for efficient CO2 electroreduction to hydrocarbons and alcohols. ACS Nano 11, 4825–4831 (2017).
Handoko, A. D. et al. Mechanistic insights into the selective electroreduction of carbon dioxide to ethylene on Cu2O-derived copper catalysts. J. Phys. Chem. C. 120, 20058–20067 (2016).
Ren, D. et al. Selective electrochemical reduction of CO2 to ethylene and ethanol on copper(i) oxide catalysts. ACS Catal. 5, 2814–2821 (2015).
De Luna, P. et al. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 1, 103–110 (2018).
Le Duff, C. S., Lawrence, M. J. & Rodriguez, P. Role of the adsorbed oxygen species in the selective electrochemical reduction of CO2 to alcohols and carbonyls on copper electrodes. Angew. Chem. Int. Ed. 56, 12919–12924 (2017).
Gao, D., Scholten, F. & Roldan Cuenya, B. Improved CO2 electroreduction performance on plasma-activated Cu catalysts via electrolyte design: halide effect. ACS Catal. 7, 5112–5120 (2017).
Kimura, K. W. et al. Controlled selectivity of CO2 reduction on copper by pulsing the electrochemical potential. ChemSusChem 11, 1781–1786 (2018).
Lim, C. F. C., Harrington, D. A. & Marshall, A. T. Altering the selectivity of galvanostatic CO2 reduction on Cu cathodes by periodic cyclic voltammetry and potentiostatic steps. Electrochim. Acta 222, 133–140 (2016).
Kumar, B. et al. Controlling the product syngas H2:CO ratio through pulsed-bias electrochemical reduction of CO2 on copper. ACS Catal. 6, 4739–4745 (2016).
Yano, J. & Yamasaki, S. Pulse-mode electrochemical reduction of carbon dioxide using copper and copper oxide electrodes for selective ethylene formation. J. Appl. Electrochem. 38, 1721 (2008).
Jermann, B. & Augustynski, J. Long-term activation of the copper cathode in the course of CO2 reduction. Electrochim. Acta 39, 1891–1896 (1994).
Shiratsuchi, R., Aikoh, Y. & Nogami, G. Pulsed electroreduction of CO2 on copper electrodes. J. Electrochem. Soc. 140, 3479–3482 (1993).
Engelbrecht, A. et al. On the electrochemical CO2 reduction at copper sheet electrodes with enhanced long-term stability by pulsed electrolysis. J. Electrochem. Soc. 165, J3059–J3068 (2018).
Velasco-Vélez, J.-J. et al. The role of the copper oxidation state in the electrocatalytic reduction of CO2 into valuable hydrocarbons. ACS Sustain. Chem. Eng. 7, 1485–1492 (2019).
Engstfeld, A. K., Maagaard, T., Horch, S., Chorkendorff, I. & Stephens, I. E. L. Polycrystalline and single-crystal Cu electrodes: influence of experimental conditions on the electrochemical properties in alkaline media. Chem. Eur. J. 24, 17743–17755 (2018).
Kim, Y.-G. et al. Surface reconstruction of pure-Cu single-crystal electrodes under CO-reduction potentials in alkaline solutions: a study by seriatim ECSTM-DEMS. J. Electroanal. Chem. 780, 290–295 (2016).
Kim, Y.-G., Baricuatro, J. H. & Soriaga, M. P. Surface reconstruction of polycrystalline Cu electrodes in aqueous KHCO3 electrolyte at potentials in the early stages of CO2 reduction. Electrocatalysis 9, 526–530 (2018).
Kim, Y.-G., Baricuatro, J. H., Javier, A., Gregoire, J. M. & Soriaga, M. P. The evolution of the polycrystalline copper surface, first to Cu(111) and then to Cu(100), at a fixed CO2RR potential: a study by operando EC-STM. Langmuir 30, 15053–15056 (2014).
Protopopoff, E. & Marcus, P. Potential–pH diagrams for hydroxyl and hydrogen adsorbed on a copper surface. Electrochim. Acta 51, 408–417 (2005).
Schouten, K. J. P., Gallent, E. P. & Koper, M. T. M. The electrochemical characterization of copper single-crystal electrodes in alkaline media. J. Electroanal. Chem. 699, 6–9 (2013).
Schouten, K. J. P. et al. Structure sensitivity of the electrochemical reduction of carbon monoxide on copper single crystals. ACS Catal. 3, 1292–1295 (2013).
Mariano, R. G., McKelvey, K., White, H. S. & Kanan, M. W. Selective increase in CO2 electroreduction activity at grain-boundary surface terminations. Science 358, 1187–1192 (2017).
Kas, R. et al. Electrochemical CO2 reduction on Cu2O-derived copper nanoparticles: controlling the catalytic selectivity of hydrocarbons. Phys. Chem. Chem. Phys. 16, 12194–12201 (2014).
Tang, W. et al. The importance of surface morphology in controlling the selectivity of polycrystalline copper for CO2 electroreduction. Phys. Chem. Chem. Phys. 14, 76–81 (2012).
Trasatti, S. & Petrii, O. A. Real surface area measurements in electrochemistry. Pure Appl. Chem. 63, 711 (1991).
Clark, E. L. et al. Standards and protocols for data acquisition and reporting for studies of the electrochemical reduction of carbon dioxide. ACS Catal. 8, 6560–6570 (2018).
Hagman, B. et al. Steps control the dissociation of CO2 on Cu(100). J. Am. Chem. Soc. 140, 12974–12979 (2018).
Hahn, C. et al. Engineering Cu surfaces for the electrocatalytic conversion of CO2: controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl Acad. Sci. USA 114, 5918–5923 (2017).
Kortlever, R., Shen, J., Schouten, K. J. P., Calle-Vallejo, F. & Koper, M. T. M. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Wuttig, A. & Surendranath, Y. Impurity ion complexation enhances carbon dioxide reduction catalysis. ACS Catal. 5, 4479–4484 (2015).
Biesinger, M. C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 49, 1325–1334 (2017).
Acknowledgements
This work was supported by the European Research Council under grant ERC-OPERANDOCAT (ERC-725915) and the German Federal Ministry of Education and Research (BMBF) under grant nos. 033RCOO4D-‘e-Ethylene’ and 03SF0523C-‘CO2EKAT’. S.K. acknowledges financial support from the Max Planck Research School for Interface Controlled Materials for Energy Conversion (IMPRS-SurMat). Funding from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany´s Excellence Strategy (EXC 2008/1 (UniSysCat)) 390540038 is also appreciated.
Author information
Authors and Affiliations
Contributions
R.M.A.A. designed the electrochemical experiments, analysed the results and wrote the manuscript. F.S. performed the quasi in situ XPS measurements and wrote the corresponding section. S.K. carried out the microscopic characterization by STM and AFM. R.R. performed the DEMS experiments and wrote the corresponding section. B.R.C. co-designed the experiments, guided and supervised the project and co-wrote the manuscript. All authors discussed the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16, Notes 1–4, Tables 1 and 2, and refs. 1–8.
Rights and permissions
About this article
Cite this article
Arán-Ais, R.M., Scholten, F., Kunze, S. et al. The role of in situ generated morphological motifs and Cu(i) species in C2+ product selectivity during CO2 pulsed electroreduction. Nat Energy 5, 317–325 (2020). https://doi.org/10.1038/s41560-020-0594-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41560-020-0594-9
This article is cited by
-
Hybrid oxide coatings generate stable Cu catalysts for CO2 electroreduction
Nature Materials (2024)
-
Ligand-modified nanoparticle surfaces influence CO electroreduction selectivity
Nature Communications (2024)
-
A surface strategy boosting the ethylene selectivity for CO2 reduction and in situ mechanistic insights
Nature Communications (2024)
-
Stability and lifetime of diffusion-trapped oxygen in oxide-derived copper CO2 reduction electrocatalysts
Nature Catalysis (2024)
-
Dynamics of bulk and surface oxide evolution in copper foams for electrochemical CO2 reduction
Communications Chemistry (2024)