Abstract
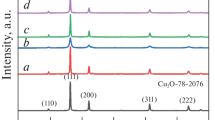
Cuprous oxide (Cu2O) microspheres were synthesized through a simple chemical method by using ascorbic acid (AA) as reducing agent at room temperature and short reaction time. The influence of the reducing agent and ammonium hydroxide (NH4OH), as well as reaction time, on the morphology, size, and crystalline phase structure of Cu2O were explored. The obtained materials were characterized by X-ray diffraction, scanning electron microscopy, nitrogen physisorption, and temperature-programmed reduction, whereas the catalytic activity was assessed using the photodegradation of methyl orange (MO). It was found that AA was responsible for the Cu2O nanoparticles self-assembling into Cu2O microspheres after 0.16 h of reaction time. Quantum theoretical calculations revealed that self-assembly is due to the hydrophilic modification of the Cu2O-nanoparticles driven by the parallel-to-surface dehydroascorbic acid chemisorption; likewise, the simulation of a mesoscopic molecular dynamics confirmed that the amphiphilic-like nature of the dehydroascorbic acid-Cu2O complexes leads to nanoparticles’ spherical morphology. The NH4OH concentration affected the porosity and crystalline phase of the microspheres. The porous Cu2O microspheres modified into well-defined Cu0 polyhedrons with the reaction time. The Cu2O microspheres exhibited high photocatalytic activity up to 76% during decolorization of MO. Two catalytic processes were distinguished: the removal of organic compound controlled by the faceting of the particles under dark conditions, and its photodegradation using visible light increased by the porosity of the Cu2O microspheres.
















Similar content being viewed by others
References
Zhang YH, Jiu BB, Gong FL, Chen JL, Zhang HL (2017) Morphology-controllable Cu2O supercrystals: facile synthesis, facet etching mechanism and comparative photocatalytic H2 production. J Alloy Compd 729:563–570
Yeo BE, Cho YS, Huh YD (2017) Evolution of the morphology of Cu2O microcrystals: cube to 50-facet polyhedron through beveled cube and rhombicuboctahedron. CrystEngComm 19:1627–1632
Yang Y, Han J, Ning X, Cao W, Xu W, Guo L (2014) Controllable morphology and conductivity of electrodeposited Cu2O thin film: effect of surfactants. ACS Appl Mater Interfaces 6:22534–22543
Huang WC, Lyu LM, Yang YC, Huang MH (2012) Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J Am Chem Soc 134:1261–1267
Bi Y, Ouyang S, Umezawa N, Cao J, Ye J (2011) Facet effect of single-crystalline Ag3PO4 sub-microcrystals on photocatalytic properties. J Am Chem Soc 133:6490–6492
Wang J, Gong J, Xiong Y, Yang J, Gao Y, Liu Y, Lu X, Tang Z (2011) Shape-dependent electrocatalytic activity of monodispersed gold nanocrystals toward glucose oxidation. Chem Commun 47:6894–6896
Lee HS, Woo CS, Youn BK, Kim S, Oh ST, Sung Y, Lee HI (2005) Bandgap modulation of TiO2 and its effect on the activity in photocatalytic oxidation of 2-isopropyl-6-methyl-4-pyrimidinol. Top Catal 35:255–260
Liu Y, Wang D, Peng Q, Chu D, Liu X, Li Y (2011) Directly assembling ligand-free ZnO Nanocrystals into three-dimensional mesoporous structures by oriented attachment. Inorg Chem 50:5841–5847
Yao KX, Yin XM, Wang TH, Zeng HC (2010) Synthesis, self-assembly, disassembly, and reassembly of two types of Cu2O nanocrystals unifaceted with 001 or 110 planes. J Am Chem Soc 132:6131–6144
Zeng QX, Xu GC, Zhang L, Lv Y (2019) Porous Cu2O microcubes derived from a metal-formate framework as photocatalyst for degradation of methyl orange. Mater Res Bull 119:110537
Chen D, Zhang X, Lee AF (2015) Synthetic strategies to nanostructured photocatalysts for CO2 reduction to solar fuels and chemicals. J Mater Chem A 3:14487–14516
Hisatomi T, Kubota J, Domen K (2014) Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem Soc Rev 43:7520–7535
Ren J, Wang W, Sun S, Zhang L, Wang L, Chang J (2011) Crystallography facet-dependent antibacterial activity: the case of Cu2O. Ind Eng Chem Res 50:10366–10369
Wan X, Wang J, Zhu L, Tang J (2014) Gas sensing properties of Cu2O and its particle size and morphology-dependent gas-detection sensitivity. J Mater Chem 2:13641–13647
Wijesundera RP (2010) Fabrication of the CuO/Cu2O heterojunction using an electrodeposition technique for solar cell applications. Semicond Sci Technol 25:45015–45019
Kim ES, Kim MC, Moon SH, Shin YK, Lee JE, Choi S, Park W (2019) Surface modified and size-controlled octahedral Cu2O nanostructured electrodes for lithium-ion batteries. J Alloy Compd 794:84–93
Zhu H, Wang J, Xu G (2009) Fast synthesis of Cu2O hollow microspheres and their application in DNA biosensor of hepatitis B. Virus Crystal Growth & Design 9:633–638
Zhang Z, Wu H, Yu Z, Song R, Qian K, Chen X, Tian J, Zhang W, Huang W (2019) Site-resolved Cu2O catalysis in the oxidation of CO. Angew Chem Int Ed 58:4276–4280
Kattel S, Ramírez PJ, Chen JG, Rodriguez JA, Liu P (2017) Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 355:1296–1299
Kim MH, Lim B, Lee EP, Xia Y (2008) Polyol synthesis of Cu2O nanoparticles: use of chloride to promote the formation of a cubic morphology. J Mater Chem 18:4069–4073
Dong C, Zhong M, Huang T, Ma M, Wortmann D, Brajdic M, Kelbassa I (2011) Photodegradation of methyl orange under visible light by micro-nano hierarchical Cu2O structure fabricated by hybrid laser processing and chemical dealloying. ACS Appl Mater Interfaces 3:4332–4338
Yu Y, Zhang L, Wang J, Yang Z, Long M, Hu N, Zhang Y (2012) Preparation of hollow porous Cu2O microspheres and photocatalytic activity under visible light irradiation. Nanoscale Res Lett 7:347–352
Deng X, Zhang Q, Zhao Q, Ma L, Ding M, Xu X (2015) Effects of architectures and H2O2 additions on the photocatalytic performance of hierarchical Cu2O nanostructures. Nanoscale Res Lett 10:8–16
Teo JJ, Chang Y, Zeng HC (2006) Fabrications of hollow nanocubes of Cu2O and Cu via reductive self-assembly of CuO nanocrystals. Langmuir 22:7369–7377
Leng M, Liu M, Zhang Y, Wang Z, Yu C, Yang X, Zhang H, Wang C (2010) Polyhedral 50-facet Cu2O microcrystals partially enclosed by 311 high-index planes: synthesis and enhanced catalytic CO oxidation activity. J Am Chem Soc 132:17084–17087
Yu H, Yu J, Liu S, Mann S (2007) Template-free hydrothermal synthesis of CuO/Cu2O composite hollow microspheres. Chem Mater 17:4327–4334
Zhang Y, Deng B, Zhang T, Gao D, Xu AW (2010) Shape effects of Cu2O polyhedral microcrystals on photocatalytic activity. J Phys Chem A 114:5073–5079
Xu H, Wang W, Zhu W (2006) Shape evolution and size-controllable synthesis of Cu2O octahedra and their morphology-dependent photocatalytic properties. J Phys Chem B 110:13829–13834
Vivas L, Chi-Duran I, Enríquez J, Barraza N, Singh DP (2019) Ascorbic acid based controlled growth of various Cu and Cu2O nanostructures. Mater Res Express 6:065033–065040
Begletsova N, Selifonova E, Chumakov A, Al-Alwani A, Zakharevich A, Chernova R, Glukhovskoy E (2018) Chemical synthesis of copper nanoparticles in aqueous solutions in the presence of anionic surfactant sodium dodecyl sulfate. Colloids Surf A 552:75–80
Chen L, Zhang D, Chen J, Zhou H, Wan H (2006) The use of CTAB to control the size of copper nanoparticles and the concentration of alkylthiols on their surface. Mater Sci Eng A 415:156–161
Mishra AK, Pradhan D (2016) Morphology controlled solution-based synthesis of Cu2O crystals for the facets-dependent catalytic reduction of highly toxic aqueous Cr(VI). Cryst Growth Des 7:3688–3698
Imangaliyeva A, Mastai Y, Seilkhanova G (2019) In situ synthesis and catalytic properties of Cu2O nanoparticles based on clay materials and polyethylene glycol. J Nanopart Res 21:97–107
Sawant S, Bhagwat A, Mahajan C (2016) Synthesis of cuprous oxide (Cu2O) nanoparticles. J Nano- Electron Phys 8:1035–1041
Thoka S, Lee AT, Huang MH (2019) Scalable synthesis of size-tunable small Cu2O nanocubes and octahedra for facet-dependent optical characterization and pseudomorphic conversion to Cu nanocrystals. ACS Sustain Chem Eng 7:10467–10476
Yu C, Shu Y, Zhou X, Ren Y, Liu Z (2018) Multi-branched Cu2O nanowires for photocatalytic degradation of methyl orange. Mater Res Express 5:035046–035054
Dan Z, Yang Y, Qin F, Wang H, Chang H (2018) Facile fabrication of Cu2O nanobelts in ethanol on nanoporous Cu and their photodegradation of methyl orange. Materials 11:446–459
Zhang J, Liu J, Peng Q, Wang X, Li Y (2006) Nearly monodisperse Cu2O and CuO nanospheres: preparation and applications for sensitive gas sensors. Chem Mater 18:867–871
Wei B, Yang N, Pang F, Ge J (2018) Cu2O–CuO hollow nanospheres as a heterogeneous catalyst for synergetic oxidation of CO. J Phys Chem C 122:19524–19531
Kumar S, Parlett CMA, Isaacs MA, Jowett DV, Douthwaite RE, McR C, Lee AF (2016) Facile synthesis of hierarchical Cu2O nanocubes as visible light photocatalysts. Appl Catal B 189:226–232
Chen L, Zhang Y, Zhu P, Zhou F, Zeng W, Lu DD, Sun R, Wong C (2015) Copper salts mediated morphological transformation of Cu2O from cubes to hierarchical flower-like or microspheres and their supercapacitors performances. Sci Rep 5:9672–9978
Zhang H, Zhu Q, Zhang Y, Wang Y, Zhao L, Yu B (2007) One-pot synthesis and hierarchical assembly of hollow Cu2O Microspheres with nanocrystals-composed porous multishell and their gas-sensing properties. Adv Func Mater 17:2766–2771
Guo B, Liu G, Zeng Y, Dong G, Wang C (2018) Rapid mineralization of methyl orange by nanocrystalline-assembled mesoporous Cu2O microspheres. Nanotechnology 29:445701
Zhang F, Dong G, Wang M, Zeng Y, Wang C (2018) Efficient removal of methyl orange using Cu2O as a dual function catalyst. Appl Surf Sci 444:559–568
Hua Q, Shang D, Zhang W, Chen K, Chang S, Ma Y, Jiang Z, Yang J, Huang W (2011) Morphological evolution of Cu2O nanocrystals in an acid solution: stability of different crystal planes. Langmuir 27:665–671
Ferrer MM, Fabris GSL, de Faria BV, Martins JBL, Moreira ML, Sambrano JR (2019) Quantitative evaluation of the surface stability and morphological changes of Cu2O particles. Heliyon 5:e2500
Accelrys.com, Version 7.0, Accelrys Software Inc. (2012)
Delley B (1990) An all-electron numerical method for solving the local density functional for polyatomic molecules. J Chem Phys 92:508–517
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799
Shenoy US, Shetty AN (2014) Simple glucose reduction route for one-step synthesis of copper nanofluids. Appl Nanosci 4:47–54
Panigrahi S, Kundu S, Ghosh SK, Nath S, Praharaj S, Basu S, Pal T (2006) Selective one-pot synthesis of copper nanorods under surfactantless condition. Polyhedron 25:1263–1269
Barrita JLS, del Sánchez MDSS (2013) Antioxidant role of ascorbic acid and his protective effects on chronic diseases. Oxid Stress Chronic Degener Dis 14:45–48
Andal V, Buvaneswari G (2017) Effect of reducing agents in the conversion of Cu2O nanocolloid to Cu nanocolloid. Eng Sci Technol 20:340–344
Kulkarni SR, Saptale SP, Borse DB, Agarwal AD (2011) Green synthesis of Ag nanoparticles using vitamin C (ascorbic acid) in a batch process. Int Conf Nanosci Eng Technol 1:88–90
Jain S, Jain A, Kachhawah P, Devra V (2015) Synthesis and size control of copper nanoparticles and their catalytic application. Trans Nonferrous Metals Soc China 25:3995–4000
Nene A (2016) Fe3O4 and Fe nanoparticles by chemical reduction of Fe3 by ascorbic acid: role of water. World J Nano Sci Eng 6:20–28
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl Chem 87:1051–1069
Liu J, Gao Z, Han H, Wu D, Xu F, Wang H, Jiang K (2012) Mesoporous Cu2O submicro-spheres, facile synthesis and the selective adsorption properties. Chem Eng J 185–186:151–159
Wu Z, Overbury SH (2015) Catalysis by materials with well-defined structures. Elsevier, Amsterdam
Susman MD, Feldman Y, Vaskevich A, Rubinstein I (2014) Chemical deposition of Cu2O nanocrystals with precise morphology control. ACS Nano 8:162–174
Shang Y, Guo L (2015) Facet-controlled synthetic strategy of Cu2O-based crystals for catalysis and sensing. Adv Sci 2:1500140–1500161
Chen K, Xue D (2012) pH-assisted crystallization of Cu2O: chemical reactions control the evolution from nanowires to polyhedra. CrystEngComm 14:8068–8075
Zhang Z, Che H, Wang Y, Gao J, Zhao L, She X, Sun J, Gunawan P, Zhong Z, Su F (2012) Facile synthesis of mesoporous Cu2O microspheres with improved catalytic property for dimethyldichlorosilane. Synth Indus Eng Chem Res 51:1264–1274
Liu B, Hu X (2020) Chapter 1 - Hollow micro and nanomaterials: synthesis and applications. Adv Nanomater Pollut Sensing Environ Catal 1:1–38
Xiong J, Wang Y, Xue Q, Wu X (2011) Synthesis of highly stable dispersions of nanosized copper particles using l-ascorbic acid. Green Chem 13:900–904
Silva N, Ramírez S, Díaz I, Garcia A, Hassan N (2019) Easy, quick, and reproducible sonochemical synthesis of CuO nanoparticles. Materials 12:804–816
Ma ZC, Wang LM, Chu DQ, Sun HM, Wang AX (2015) Template-free synthesis of complicated double-wall Cu2O hollow spheres with enhanced visible photocatalytic activities. RSC Adv 5:8223–8227
Ng CHB, Fan WY (2006) Shape evolution of Cu2O nanostructures via kinetic and thermodynamic controlled growth. J Phys Chem B 110:20801–20807
Duan X, Gao R, Zhang Y, Jian Z (2011) Synthesis of sea urchin-like cuprous oxide with hollow glass microspheres as cores and its preliminary application as a photocatalyst. Mater Lett 65:3625–3628
Acknowledgements
The authors acknowledge financial support from Mexican Institute of Petroleum (IMP), Project-D.61048 and especially to the postgraduate area. Particularly, D. A. Prado-Chay is very grateful to CONACYT for the grant to support his postgraduate studies.
Funding
This research is a product of Project D.61048 “Desarrollo de un proceso catalítico para la obtención de compuestos aromáticos a partir de lignina” funded by Mexican Institute of Petroleum.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Research Involving Human and/or Animals rights
The research involved no human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prado-Chay, D.A., Cortés-Jácome, M.A., Angeles-Chávez, C. et al. Synthesis and Photocatalytic Activity of Cu2O Microspheres upon Methyl Orange Degradation. Top Catal 63, 586–600 (2020). https://doi.org/10.1007/s11244-020-01256-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01256-5