Abstract
Anti-fungal hydrogel wound dressings were prepared using the ion cross-linked poly (AA-co-AAm)/PVA/Cloisite 15A nanocomposite hydrogels which were loaded by allicin. The allicin is the main anti-fungal and anti-biotic material of the garlic and it was extracted from fresh garlic pulps following a simple method. The extracted allicin was then loaded on synthesized hydrogel films by immersing the hydrogel films in allicin solutions with different concentrations. To investigate the inhibiting effect of the allicin on fungal specie, 5 different kinds of fungi were chosen and were cultured following the standard methods. The obtained results showed that the ion cross-linked nanocomposite hydrogel film with 5.33 mg/ml of allicin content has the best efficiency on inhibition of the fungus specie. Also it is confirmed that, in an optimum concentration of loaded allicin, the release behavior of the hydrogel is the best which means a low initial burst release and efficient time of releasing. It should be noted that, the chemical safety of the synthesized hydrogel films was evaluated using a UV-Vis method and the obtained results confirmed that there is no release of monomers from the hydrogel samples.






Similar content being viewed by others
References
Caló E, Khutoryanskiy VV (2015) Biomedical applications of hydrogels: a review of patents and commercial products. Eur Polym J 65:252–267
Wang M, Xu L, Hu H, Zhai M, Peng J, Nho Y, Li J, Wei G (2007) Radiation synthesis of PVP/CMC hydrogels as wound dressing. Nucl Instrum Methods Phys Res, Sect B 265:385–389
Sweeney IR, Miraftab M, Collyer G (2012) A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int Wound J 9:601–612
Lian Z, Ye L (2015) Synthesis and properties of carboxylated poly (vinyl alcohol) hydrogels for wound dressings. J Polym Res 22:72
Lee Y-H, Chang J-J, Yang M-C, Chien C-T, Lai W-F (2012) Acceleration of wound healing in diabetic rats by layered hydrogel dressing. Carbohydr Polym 88:809–819
Sung JH, Hwang M-R, Kim JO, Lee JH, Kim YI, Kim JH, Chang SW, Jin SG, Kim JA, Lyoo WS (2010) Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int J Pharm 392:232–240
Ailincai D, Marin L, Morariu S, Mares M, Bostanaru A-C, Pinteala M, Simionescu BC, Barboiu M (2016) Dual crosslinked iminoboronate-chitosan hydrogels with strong antifungal activity against Candida planktonic yeasts and biofilms. Carbohydr Polym 152:306–316
Wright J, Lam K, Hansen D, Burrell R (1999) Efficacy of topical silver against fungal burn wound pathogens. Am J Infect Control 27:344–350
Zumbuehl A, Ferreira L, Kuhn D, Astashkina A, Long L, Yeo Y, Iaconis T, Ghannoum M, Fink GR, Langer R (2007) Antifungal hydrogels. Proc Natl Acad Sci 104:12994–12998
Zaugg C, Monod M, Weber J, Harshman K, Pradervand S, Thomas J, Bueno M, Giddey K, Staib P (2009) Gene expression profiling in the human pathogenic dermatophyte Trichophyton rubrum during growth on proteins. Eukaryot Cell 8:241–250
Weitzman I, Summerbell RC (1995) The dermatophytes. Clin Microbiol Rev 8:240–259
Gräser Y, Scott J, Summerbell R (2008) The new species concept in dermatophytes—a polyphasic approach. Mycopathologia 166:239
Guo J, Brosnan B, Furey A, Arendt E, Murphy P, Coffey A (2012) Antifungal activity of Lactobacillus against Microsporum canis, Microsporum gypseum and Epidermophyton floccosum. Bioengineered 3:104–113
Yamada T, Nozawa Y (1979) An unusual lipid in the human pathogenic fungus Epidermophyton floccosum, Biochimica et Biophysica Acta (BBA)-lipids and lipid. Metabolism 574:433–439
Wu Y, Yang J, Yang F, Liu T, Leng W, Chu Y, Jin Q (2009) Recent dermatophyte divergence revealed by comparative and phylogenetic analysis of mitochondrial genomes. BMC Genomics 10:238
Ginter-Hanselmayer G, Smolle J, Gupta A (2004) Itraconazole in the treatment of tinea capitis caused by Microsporum canis: experience in a large cohort. Pediatr Dermatol 21:499–502
Shafiee S, Khosravi AR, Ashrafi Tamai I (2014) Comparative study of Microsporum canis isolates by DNA fingerprinting. Mycoses 57:507–512
O’Gorman CM, Fuller HT, Dyer PS (2009) Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471
Gow NA, Yadav B (2017) Microbe profile: Candida albicans: a shape-changing, opportunistic pathogenic fungus of humans. Microbiology 163:1145–1147
Erdogan A, Rao SS (2015) Small intestinal fungal overgrowth. Current gastroenterology reports 17:16
Martins N, Ferreira IC, Barros L, Silva S, Henriques M (2014) Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 177:223–240
Dismukes WE (2000) Introduction to antifungal drugs. Clin Infect Dis:653–657
Ameen M (2010) Epidemiology of superficial fungal infections. Clin Dermatol 28:197–201
Arif T, Bhosale J, Kumar N, Mandal T, Bendre R, Lavekar G, Dabur R (2009) Natural products–antifungal agents derived from plants. J Asian Nat Prod Res 11:621–638
Jansen H, Müller B, Knobloch K (1987) Allicin characterization and its determination by HPLC. Planta Med 53:559–562
Iwalokun B, Ogunledun A, Ogbolu D, Bamiro S, Jimi-Omojola J (2004) In vitro antimicrobial properties of aqueous garlic extract against multidrug-resistant bacteria and Candida species from Nigeria. J Med Food 7:327–333
Bayan L, Koulivand PH, Gorji A (2014) Garlic: a review of potential therapeutic effects. Avicenna journal of phytomedicine 4:1
Chong K, Zamora MP, Tilakawardane DA, Buckely N, Rego JA, Liu Y (2015) Investigation of allicin stability in aqueous garlic extract by high performance liquid chromatography method. Journal of Scientific Research and Reports 4:590–598
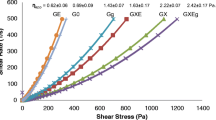
A. Olad, M. Eslamzadeh, A. Mirmohseni, Ion crosslinked poly (acrylic acid-co-acrylamide)/poly (vinyl alcohol)/Cloisite 15 a nanocomposite hydrogels as potential wound dressing films: effect of clay content on water absorption kinetic and mechanical properties, Polym Compos
N. Mansor, H.J. Herng, S.J. Samsudin, S. Sufian, Y. Uemura, Quantification and characterization of allicin in garlic extract, journal of medical and bioengineering Vol, 5 (2016)
Ilić DP, Nikolić VD, Nikolić LB, Stanković MZ, Stanojević LP, Cakić MD (2011) Allicin and related compounds: biosynthesis, synthesis and pharmacological activity. Facta universitatis-series: Physics, Chemistry and Technology 9:9–20
M. Arzanlou, S. Bohlooli, M. Ranjbar Omid, Purification of allicin from garlic extract using semi-preparative high performance liquid chromatography, Jundishapur journal of natural pharmaceutical products, 10 (2015)
Sariri R, Aliakbar A, Mesdarghi GB, Rezazadeh A (2002) Spectrophotometric assay of Allicin in Iranian garlic products. Asian J Chem 14:1197
Azhar FF, Olad A (2014) A study on sustained release formulations for oral delivery of 5-fluorouracil based on alginate–chitosan/montmorillonite nanocomposite systems. Appl Clay Sci 101:288–296
Talukdar MM, Michoel A, Rombaut P, Kinget R (1996) Comparative study on xanthan gum and hydroxypropylmethyl cellulose as matrices for controlled-release drug delivery I. compaction and in vitro drug release behaviour. Int J Pharm 129:233–241
Guyot M, Fawaz F (2000) Design and in vitro evaluation of adhesive matrix for transdermal delivery of propranolol. Int J Pharm 204:171–182
Serra L, Doménech J, Peppas NA (2006) Drug transport mechanisms and release kinetics from molecularly designed poly (acrylic acid-g-ethylene glycol) hydrogels. Biomaterials 27:5440–5451
Costa P, Lobo JMS (2001) Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13:123–133
Higuchi T (1963) Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci 52:1145–1149
Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA (1983) Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm 15:25–35
Acknowledgements
The financial support of this work by the University of Tabriz is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Olad, A., Eslamzadeh, M., Katiraee, F. et al. Evaluation of in vitro anti-fungal properties of allicin loaded ion cross-linked poly (AA-co-AAm)/PVA/Cloisite 15A Nanocomposite hydrogel films as wound dressing materials. J Polym Res 27, 100 (2020). https://doi.org/10.1007/s10965-020-02072-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02072-x