Abstract
Purpose
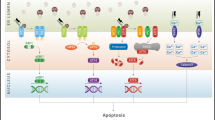
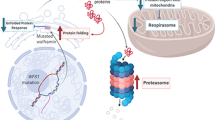
Wolfram syndrome (WS) is a rare disorder caused by mutations in WFS1 that is characterized by diabetes mellitus, optic atrophy, sensorineural deafness, diabetes insipidus, and neurodegeneration. This disease is usually inherited as an autosomal recessive trait, but an autosomal dominant form has been reported. WFS1 encodes a transmembrane protein, which is a maintenance component of endoplasmic homeostasis. These dominant mutations were thought to increase endoplasmic reticulum (ER) stress. Recent studies suggest that 4-phenylbutyrate (PBA) and valproate (VPA) reduce ER stress. The objective of this study was to analyze the effect of PBA and VPA on dominant WFS1 mutants in vitro.
Methods
We determined whether dominant WFS1 mutants (p.His313Tyr, p.Trp314Arg, p.Asp325_Ile328del, p.Glu809Lys, and p.Glu864Lys) have the dominant negative effect using a luciferase assay of ER stress response element marker as ER stress. Moreover, the rescue of cell apoptosis induced by dominant WFS1 mutants following treatment with PBA or VPA was determined by quantitative real-time PCR of C/EBP homologous protein (CHOP) mRNA expression.
Results
These mutants showed the dominant negative effect on the wild-type WFS1. In addition, the levels of ER stress and CHOP mRNA were significantly elevated by all dominant WFS1 mutants. After treatment with PBA or VPA, ER stress and cell apoptosis were reduced in each mutant.
Conclusions
PBA and VPA could reduce the ER stress and cell apoptosis caused by dominant WFS1 mutants.








Similar content being viewed by others
References
Hofmann S, Philbrook C, Gerbitz KD, Bauer MF (2003) Wolfram syndrome: structural and functional analyses of mutant and wild-type wolframin, the WFS1 gene product. Hum Mol Genet 12:2003–2012. https://doi.org/10.1093/hmg/ddg214
Morikawa S, Tajima T, Nakamura A, Ishizu K, Ariga T (2017) A novel heterozygous mutation of the WFS1 gene leading to constitutive endoplasmic reticulum stress is the cause of Wolfram syndrome. Pediatr Diabetes 18:934–941. https://doi.org/10.1111/pedi.12513
Rigoli L, Lombardo F, Di Bella C (2001) Wolfram syndrome and WFS1 gene. Clin Genet 79:103–117. https://doi.org/10.1111/j.1399-0004.2010.01522.x
Matsunaga K, Tanabe K, Inoue H et al (2014) Wolfram syndrome in the Japanese population; molecular analysis of WFS1 gene and characterization of clinical features. PLoS ONE 9:e106906. https://doi.org/10.1371/journal.pone.0106906
Urano F (2016) Wolfram syndrome: diagnosis, management, and treatment. Curr Diab Rep 16:6. https://doi.org/10.1007/s11892-015-0702-6
Bonnycastle LL, Chines PS, Hara T et al (2013) Autosomal dominant diabetes arising from a Wolfram syndrome 1 mutation. Diabetes 62:3943–3950. https://doi.org/10.2337/db13-0571
De Franco E, Flanagan SE, Yagi T et al (2017) Dominant ER Stress-Inducing WFS1 mutations underlie a genetic syndrome of neonatal/infancy-onset diabetes, congenital sensorineural deafness, and congenital cataracts. Diabetes 66:2044–2053. https://doi.org/10.2337/db16-1296
Eiberg H, Hansen L, Kjer B et al (2006) Autosomal dominant optic atrophy associated with hearing impairment and impaired glucose regulation caused by a missense mutation in the WFS1 gene. J Med Genet 43:435–440. https://doi.org/10.1136/jmg.2005.034892
Hansen L, Eiberg H, Barrett T, Bek T, Kjærsgaard P, Tranebjærg L, Rosenberg T (2005) Mutation analysis of the WFS1 gene in seven danish Wolfram syndrome families; four new mutations identified. Eur J Hum Genet 13:1275–1284. https://doi.org/10.1038/sj.ejhg.5201491
Cryns K, Thys S, Van Laer L et al (2003) The WFS1 gene, responsible for low frequency sensorineural hearing loss and Wolfram syndrome, is expressed in a variety of inner ear cells. Histochem Cell Biol 119:247–256
Fukuoka H, Kanda Y, Ohta S, Usami S (2017) Mutations in the WFS1 gene are a frequent cause of autosomal dominant nonsyndromic low-frequency hearing loss in Japanese. J Hum Genet 52:510–515. https://doi.org/10.1007/s10038-007-0144-3
Pallotta MT, Tascini G, Crispoldi R, Orabona C, Mondanelli G, Grohmann U, Esposito S (2019) Wolfram syndrome, a rare neurodegenerative disease: from pathogenesis to future treatment perspectives. J Transl Med 17:238. https://doi.org/10.1186/s12967-019-1993-1
Fonseca SG, Burcin M, Gromada J, Urano F (2009) Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol 9:763–770. https://doi.org/10.1016/j.coph.2009.07.003
Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, Urano F (2005) WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic β-cells. J Biol Chem 280:39609–39615. https://doi.org/10.1074/jbc.M507426200
Kolb PS, Ayaub EA, Zhou W, Yum V, Dickhout JG, Ask K (2015) The therapeutic effects of 4-phenylbutyric acid in maintaining proteostasis. Int J Bio Cell Biol 61:45–52. https://doi.org/10.1016/j.biocel.2015.01.015
Yam GH, Gaplovska-Kysela K, Zuber C, Roth J (2007) Sodium 4-phenylbutyrate acts as a chemical chaperone on misfolded myocilin to rescue cells from endoplasmic reticulum stress and apoptosis. Invest Opthtalmo Vis Sci 48:1683–1690. https://doi.org/10.1167/iovs.06-0943
Zeitlin PL, Diener-West M, Rubenstein RC, Boyle MP, Lee CKK, Brass-Ernst L (2002) Evidence of CFTR function in cystic fibrosis after systemic administration of 4-phenylbutyrate. Mol Ther 6:119–126. https://doi.org/10.1006/mthe.2002.0639
Huang A, Young TL, Dang VT, Shi Y, McAlpine CS, Werstuck GH (2017) 4-phenylbutyrate and valproate treatment attenuates the progression of atherosclerosis and stabilizes existing plaques. Atherosclerosis 266:103–112. https://doi.org/10.1016/j.atherosclerosis.2017.09.034
Kim DS, Li B, Rhew KY et al (2012) The regulatory mechanism of 4-phenylbutyric acid against ER stress-induced autophagy in human gingival fibroblasts. Arch Pharmacal Res 35:1269–1278. https://doi.org/10.1007/s12272-012-0718-2
Shang L, Hua H, Foo K et al (2014) β-cell dysfunction due to increased ER stress in a stem cell model of Wolfram syndrome. Diabetes 63:923–933. https://doi.org/10.2337/db13-0717
Cadavez L, Montane J, Alcarraz-Vizán G, Visa M, Vidal-Fàbrega L, Servitja J-M, Novials A (2014) Chaperones ameliorate beta cell dysfunction associated with human islet amyloid polypeptide overexpression. PLoS ONE 9:e101797. https://doi.org/10.1371/journal.pone.0101797
Kakiuchi C, Ishigaki S, Oslowski CM, Fonseca SG, Kato T, Urano F (2009) Valproate, a mood stabilizer, induces WFS1 expression and modulates its interaction with ER stress protein GRP94. PLoS ONE 4:e4134. https://doi.org/10.1371/journal.pone.0004134
Kim AJ, Shi Y, Austin RC, Werstuck GH (2005) Valproate protects cells from ER stress-induced lipid accumulation and apoptosis by inhibiting glycogen synthase kinase-3. J Cell Sci 118:89–99. https://doi.org/10.1242/jcs.01562
Li Z, Wu F, Zhang X (2017) Valproate attenuates endoplasmic reticulum stress-induced apoptosis in SH-SY5Y cells via the AKT/GSK3β signaling pathway. Int J Mol Sci 18:315. https://doi.org/10.3390/ijms18020315
Huang S, Zhu M, Wu W et al (2014) Valproate pretreatment protects pancreatic β-cells from palmitate-induced ER stress and apoptosis by inhibiting glycogen synthase kinase-3β. J Biomed Sci 21:38. https://doi.org/10.1186/1423-0127-21-38
Terasmaa A, Soomets U, Oflijan J (2011) Wfs1 mutation makes mice sensitive to insulin-like effect of acute valproic acid and resistant to streptozocin. J Physiol Biochem 67:381–390. https://doi.org/10.1007/s13105-011-0088-0
Fonseca SG, Ishigaki S, Oslowski CM et al (2010) Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Inverst 120:744–755. https://doi.org/10.1172/JCI39678
Wu J, Rutkowski DT, Dubois M et al (2007) ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13:351–364. https://doi.org/10.1016/j.devcel.2007.07.005
Nishitoh H (2011) CHOP is a multifunctional transcription factor in the ER stress response. J Biochem 151:217–219. https://doi.org/10.1093/jb/mvr143
Ueda K, Kawano J, Takeda K et al (2005) Endoplasmic reticulum stress induces Wfs1 gene expression in pancreatic β-cells via transcriptional activation. Eur J Endocrinol 153:167–176. https://doi.org/10.1530/eje.1.01945
Yamada T, Ishihara H, Tamura A et al (2006) WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic β-cells. Hum Mol Genet 15:1600–1609. https://doi.org/10.1093/hmg/ddl081
Hara T, Mahadevan J, Kanekura K et al (2014) Calcium efflux from the endoplasmic reticulum leads to β-cell death. Endocrinology 155:758–768. https://doi.org/10.1210/en.2013-1519
Acknowledgements
We are grateful to Dr. F. Urano (Washington University School of Medicine) for the kind gift of the ERSE luciferase plasmid and WFS1 FLAG expression plasmid, and Dr. K.Tanabe (Yamaguchi University, Japan) for the kind gift of the WFS1 expression plasmid, Dr. S. Oyadomari (Tokushima University, Japan) for the kind gift of the ATF6α expression vector and Dr.N. Kotani, Dr J. Nakae (Keio University, Japan) and Dr. T. Kitamura (Gunma University, Japan) for the kind gift of the MIN6 cell line.
Funding
None declared.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Khishigjargal Batjargal, Toshihiro Tajima and Eriko Jimbo. The first draft of the manuscript was written by Khishigjargal Batjargal. Toshihiro Tajima and Eriko Jimbo commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies involving human participants performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Batjargal, K., Tajima, T., Jimbo, E.F. et al. Effect of 4-phenylbutyrate and valproate on dominant mutations of WFS1 gene in Wolfram syndrome. J Endocrinol Invest 43, 1317–1325 (2020). https://doi.org/10.1007/s40618-020-01228-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01228-2